Inflammation of Paediatric Pulmonary Diseases
Cell type proportions analysis: annotation level 3
Jovana Maksimovic
December 31, 2024
Last updated: 2024-12-31
Checks: 7 0
Knit directory: paed-inflammation-CITEseq/
This reproducible R Markdown analysis was created with workflowr (version 1.7.1). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20240216) was run prior to running
the code in the R Markdown file. Setting a seed ensures that any results
that rely on randomness, e.g. subsampling or permutations, are
reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version b812210. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for
the analysis have been committed to Git prior to generating the results
(you can use wflow_publish or
wflow_git_commit). workflowr only checks the R Markdown
file, but you know if there are other scripts or data files that it
depends on. Below is the status of the Git repository when the results
were generated:
Ignored files:
Ignored: .Rhistory
Ignored: .Rproj.user/
Ignored: analysis/obsolete/
Ignored: data/C133_Neeland_batch1/
Ignored: data/C133_Neeland_merged/
Ignored: output/dge_analysis/obsolete/
Ignored: renv/library/
Ignored: renv/staging/
Untracked files:
Untracked: analysis/14.0_DGE_analysis_T-cells.Rmd
Untracked: analysis/15.2_proportions_analysis_ann_level_3_macrophages.Rmd
Untracked: analysis/17.0_Figure_3.Rmd
Untracked: analysis/17.0_Figure_4.Rmd
Untracked: analysis/17.0_Figure_5.Rmd
Untracked: analysis/figure/
Untracked: broad_markers_seurat.csv
Untracked: code/background_job.R
Untracked: code/reverse_modifier_severity_comparisons.sh
Untracked: data/intermediate_objects/CD4 T cells.CF_samples.fit.rds
Untracked: data/intermediate_objects/CD4 T cells.all_samples.fit.rds
Untracked: data/intermediate_objects/CD8 T cells.CF_samples.fit.rds
Untracked: data/intermediate_objects/CD8 T cells.all_samples.fit.rds
Untracked: data/intermediate_objects/DC cells.CF_samples.fit.rds
Untracked: data/intermediate_objects/DC cells.all_samples.fit.rds
Untracked: data/intermediate_objects/T cells.CF_samples.fit.rds
Untracked: data/intermediate_objects/T cells.all_samples.fit.rds
Untracked: output/dge_analysis/T cells/
Unstaged changes:
Modified: .gitignore
Modified: analysis/06.0_azimuth_annotation.Rmd
Modified: analysis/09.0_integrate_cluster_macro_cells.Rmd
Modified: analysis/13.1_DGE_analysis_macro-alveolar.Rmd
Deleted: analysis/14.0_proportions_analysis_ann_level_1.Rmd
Deleted: analysis/14.1_proportions_analysis_ann_level_3_non-macrophages.Rmd
Deleted: analysis/14.2_proportions_analysis_ann_level_3_macrophages.Rmd
Modified: analysis/15.0_Figure_1.Rmd
Modified: analysis/15.0_proportions_analysis_ann_level_1.Rmd
Modified: analysis/16.0_Figure_2.Rmd
Modified: analysis/17.0_Supplementary_Figure_ADTs.Rmd
Modified: code/utility.R
Modified: data/cluster_annotations/marker_proteins_TNK_supp.xlsx
Modified: data/cluster_annotations/marker_proteins_macrophages_supp.xlsx
Modified: data/cluster_annotations/marker_proteins_other_supp.xlsx
Modified: data/cluster_annotations/seurat_markers_all_cells.rds
Modified: data/intermediate_objects/macro-alveolar.CF_samples.fit.rds
Modified: data/intermediate_objects/macro-alveolar.all_samples.fit.rds
Modified: output/dge_analysis/macro-alveolar/ORA.FIBROSIS.CF.IVAvCF.NO_MOD.csv
Modified: output/dge_analysis/macro-alveolar/ORA.FIBROSIS.CF.IVAvNON_CF.CTRL.csv
Modified: output/dge_analysis/macro-alveolar/ORA.FIBROSIS.CF.NO_MOD.SvCF.NO_MOD.M.csv
Modified: output/dge_analysis/macro-alveolar/ORA.FIBROSIS.CF.NO_MODvNON_CF.CTRL.csv
Modified: output/dge_analysis/macro-alveolar/ORA.GO.CF.IVAvCF.NO_MOD.csv
Modified: output/dge_analysis/macro-alveolar/ORA.GO.CF.IVAvNON_CF.CTRL.csv
Modified: output/dge_analysis/macro-alveolar/ORA.GO.CF.NO_MOD.SvCF.NO_MOD.M.csv
Modified: output/dge_analysis/macro-alveolar/ORA.GO.CF.NO_MODvNON_CF.CTRL.csv
Modified: output/dge_analysis/macro-alveolar/ORA.HALLMARK.CF.IVAvCF.NO_MOD.csv
Modified: output/dge_analysis/macro-alveolar/ORA.HALLMARK.CF.IVAvNON_CF.CTRL.csv
Modified: output/dge_analysis/macro-alveolar/ORA.HALLMARK.CF.NO_MOD.SvCF.NO_MOD.M.csv
Modified: output/dge_analysis/macro-alveolar/ORA.HALLMARK.CF.NO_MODvNON_CF.CTRL.csv
Modified: output/dge_analysis/macro-alveolar/ORA.REACTOME.CF.IVAvCF.NO_MOD.csv
Modified: output/dge_analysis/macro-alveolar/ORA.REACTOME.CF.IVAvNON_CF.CTRL.csv
Modified: output/dge_analysis/macro-alveolar/ORA.REACTOME.CF.NO_MOD.SvCF.NO_MOD.M.csv
Modified: output/dge_analysis/macro-alveolar/ORA.REACTOME.CF.NO_MODvNON_CF.CTRL.csv
Modified: output/dge_analysis/macro-alveolar/ORA.WP.CF.IVAvCF.NO_MOD.csv
Modified: output/dge_analysis/macro-alveolar/ORA.WP.CF.IVAvNON_CF.CTRL.csv
Modified: output/dge_analysis/macro-alveolar/ORA.WP.CF.NO_MOD.SvCF.NO_MOD.M.csv
Modified: output/dge_analysis/macro-alveolar/ORA.WP.CF.NO_MODvNON_CF.CTRL.csv
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were
made to the R Markdown
(analysis/15.1_proportions_analysis_ann_level_3_non-macrophages.Rmd)
and HTML
(docs/15.1_proportions_analysis_ann_level_3_non-macrophages.html)
files. If you’ve configured a remote Git repository (see
?wflow_git_remote), click on the hyperlinks in the table
below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | b812210 | Jovana Maksimovic | 2024-12-31 | wflow_publish("analysis/15.1_proportions_analysis_ann_level_3_non-macrophages.Rmd") |
Load libraries
suppressPackageStartupMessages({
library(SingleCellExperiment)
library(edgeR)
library(tidyverse)
library(ggplot2)
library(Seurat)
library(glmGamPoi)
library(dittoSeq)
library(clustree)
library(AnnotationDbi)
library(org.Hs.eg.db)
library(glue)
library(speckle)
library(patchwork)
library(paletteer)
library(tidyHeatmap)
library(here)
})
set.seed(42)
options(scipen=999)
options(future.globals.maxSize = 6500 * 1024^2)Load Data
files <- list.files(here("data/C133_Neeland_merged"),
pattern = "C133_Neeland_full_clean.*(t_cells|other_cells)_annotated_diet.SEU.rds",
full.names = TRUE)
seuLst <- lapply(files, function(f) readRDS(f))
seu <- merge(seuLst[[1]],
y = c(seuLst[[2]]))
seuAn object of class Seurat
19973 features across 29198 samples within 1 assay
Active assay: RNA (19973 features, 0 variable features) used (Mb) gc trigger (Mb) max used (Mb)
Ncells 11753109 627.7 20174885 1077.5 14082532 752.1
Vcells 138257994 1054.9 375135597 2862.1 328405239 2505.6Analyse Cell type proportions
# Differences in cell type proportions
props <- getTransformedProps(clusters = seu$ann_level_3,
sample = seu$sample.id, transform="asin")
props$Proportions %>% knitr::kable()| sample_1.1 | sample_15.1 | sample_16.1 | sample_17.1 | sample_18.1 | sample_19.1 | sample_2.1 | sample_20.1 | sample_21.1 | sample_22.1 | sample_23.1 | sample_24.1 | sample_25.1 | sample_26.1 | sample_27.1 | sample_28.1 | sample_29.1 | sample_3.1 | sample_30.1 | sample_31.1 | sample_32.1 | sample_33.1 | sample_34.1 | sample_34.2 | sample_34.3 | sample_35.1 | sample_35.2 | sample_36.1 | sample_36.2 | sample_37.1 | sample_37.2 | sample_37.3 | sample_38.1 | sample_38.2 | sample_38.3 | sample_39.1 | sample_39.2 | sample_4.1 | sample_40.1 | sample_41.1 | sample_42.1 | sample_43.1 | sample_5.1 | sample_6.1 | sample_7.1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B cells | 0.1856061 | 0.0482375 | 0.0311111 | 0.2309942 | 0.0173077 | 0.1276596 | 0.2475845 | 0.0404984 | 0.1284635 | 0.0711111 | 0.2189219 | 0.0332005 | 0.0326531 | 0.0909091 | 0.0196850 | 0.0164474 | 0.2936345 | 0.1896552 | 0.0143198 | 0.0182927 | 0.0398010 | 0.1157703 | 0.0205128 | 0.0000000 | 0.0240964 | 0.1489002 | 0.0853175 | 0.1621622 | 0.2230392 | 0.1123596 | 0.2140351 | 0.1269036 | 0.2093023 | 0.1792453 | 0.2611276 | 0.2583170 | 0.1071429 | 0.0513532 | 0.3424487 | 0.0784857 | 0.0523504 | 0.1044341 | 0.0686695 | 0.0673077 | 0.2083994 |
| CD4 T cells | 0.0492424 | 0.1001855 | 0.1288889 | 0.0628655 | 0.0538462 | 0.0638298 | 0.0772947 | 0.1526480 | 0.1360202 | 0.1555556 | 0.1034103 | 0.1049137 | 0.1632653 | 0.1553030 | 0.0944882 | 0.0855263 | 0.0513347 | 0.0758621 | 0.3166269 | 0.2256098 | 0.1442786 | 0.1741112 | 0.0461538 | 0.0232558 | 0.0321285 | 0.0913706 | 0.0853175 | 0.0291060 | 0.1188725 | 0.1067416 | 0.1508772 | 0.1675127 | 0.1627907 | 0.1792453 | 0.1186944 | 0.0861057 | 0.1203416 | 0.0458015 | 0.1796147 | 0.1135734 | 0.1191239 | 0.0705951 | 0.1845494 | 0.1483516 | 0.1671949 |
| CD4 T-IFN | 0.0151515 | 0.0426716 | 0.0133333 | 0.0043860 | 0.0057692 | 0.0425532 | 0.0000000 | 0.0000000 | 0.0176322 | 0.0088889 | 0.0044004 | 0.0212483 | 0.0122449 | 0.0189394 | 0.0118110 | 0.0065789 | 0.0000000 | 0.0068966 | 0.0095465 | 0.0162602 | 0.0199005 | 0.0109389 | 0.0051282 | 0.0000000 | 0.0080321 | 0.0067682 | 0.0337302 | 0.0000000 | 0.0049020 | 0.0000000 | 0.0070175 | 0.0152284 | 0.0232558 | 0.0566038 | 0.0178042 | 0.0039139 | 0.0108696 | 0.0013879 | 0.0018645 | 0.0166205 | 0.0267094 | 0.0049592 | 0.0021459 | 0.0109890 | 0.0055468 |
| CD4 T-naïve | 0.0037879 | 0.0204082 | 0.0044444 | 0.0073099 | 0.0173077 | 0.0212766 | 0.0072464 | 0.0186916 | 0.0226700 | 0.0177778 | 0.0341034 | 0.0073041 | 0.0122449 | 0.0378788 | 0.0078740 | 0.0131579 | 0.0164271 | 0.0068966 | 0.0588703 | 0.0345528 | 0.0049751 | 0.0218778 | 0.0051282 | 0.0000000 | 0.0000000 | 0.0135364 | 0.0059524 | 0.0000000 | 0.0171569 | 0.0168539 | 0.0105263 | 0.0050761 | 0.0000000 | 0.0094340 | 0.0089021 | 0.0039139 | 0.0326087 | 0.0034698 | 0.0298322 | 0.0092336 | 0.0106838 | 0.0099183 | 0.0407725 | 0.0096154 | 0.0657686 |
| CD4 T-NFKB | 0.0000000 | 0.0074212 | 0.0044444 | 0.0043860 | 0.0057692 | 0.0000000 | 0.0060386 | 0.0093458 | 0.0604534 | 0.0311111 | 0.0187019 | 0.0099602 | 0.0163265 | 0.0189394 | 0.0039370 | 0.0065789 | 0.0205339 | 0.0034483 | 0.0031822 | 0.0121951 | 0.0348259 | 0.0638104 | 0.0256410 | 0.0155039 | 0.0120482 | 0.0135364 | 0.0138889 | 0.0270270 | 0.0232843 | 0.0674157 | 0.0456140 | 0.0558376 | 0.0116279 | 0.0188679 | 0.0178042 | 0.0704501 | 0.0535714 | 0.0020819 | 0.0186451 | 0.0572484 | 0.0058761 | 0.0049592 | 0.0107296 | 0.0082418 | 0.0158479 |
| CD4 T-reg | 0.0113636 | 0.0055659 | 0.0488889 | 0.0131579 | 0.0134615 | 0.0000000 | 0.0060386 | 0.0218069 | 0.0201511 | 0.0266667 | 0.0176018 | 0.0126162 | 0.0244898 | 0.0151515 | 0.0039370 | 0.0131579 | 0.0041068 | 0.0034483 | 0.0310263 | 0.0223577 | 0.0248756 | 0.0255242 | 0.0051282 | 0.0155039 | 0.0080321 | 0.0186125 | 0.0317460 | 0.0145530 | 0.0220588 | 0.0280899 | 0.0491228 | 0.0761421 | 0.0232558 | 0.0377358 | 0.0326409 | 0.0136986 | 0.0100932 | 0.0034698 | 0.0142946 | 0.0073869 | 0.0133547 | 0.0087515 | 0.0171674 | 0.0109890 | 0.0206022 |
| CD4 T-rm | 0.0037879 | 0.0092764 | 0.0133333 | 0.0043860 | 0.0076923 | 0.0000000 | 0.0024155 | 0.0093458 | 0.0075567 | 0.0088889 | 0.0066007 | 0.0053121 | 0.0040816 | 0.0037879 | 0.0078740 | 0.0164474 | 0.0041068 | 0.0034483 | 0.0023866 | 0.0060976 | 0.0000000 | 0.0054695 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0016920 | 0.0019841 | 0.0000000 | 0.0024510 | 0.0280899 | 0.0210526 | 0.0152284 | 0.0232558 | 0.0094340 | 0.0148368 | 0.0039139 | 0.0069876 | 0.0034698 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0472103 | 0.0123626 | 0.0095087 |
| CD8 T-GZMK | 0.0075758 | 0.0278293 | 0.0933333 | 0.0511696 | 0.0365385 | 0.0000000 | 0.0181159 | 0.0186916 | 0.0352645 | 0.0133333 | 0.0044004 | 0.0112882 | 0.0612245 | 0.0530303 | 0.0157480 | 0.0230263 | 0.0205339 | 0.0103448 | 0.0620525 | 0.0508130 | 0.0199005 | 0.0109389 | 0.0051282 | 0.0000000 | 0.0000000 | 0.0406091 | 0.0158730 | 0.0000000 | 0.0036765 | 0.0224719 | 0.0140351 | 0.0203046 | 0.0116279 | 0.0188679 | 0.0089021 | 0.0019569 | 0.0139752 | 0.0201249 | 0.0870106 | 0.0101570 | 0.0245726 | 0.0347141 | 0.0171674 | 0.0164835 | 0.0237718 |
| CD8 T-inflammasome | 0.0303030 | 0.0723562 | 0.0977778 | 0.0467836 | 0.1230769 | 0.0425532 | 0.0229469 | 0.1651090 | 0.0906801 | 0.1377778 | 0.0143014 | 0.0836653 | 0.0693878 | 0.0833333 | 0.0708661 | 0.1085526 | 0.0164271 | 0.0482759 | 0.1097852 | 0.1646341 | 0.0845771 | 0.1248861 | 0.0358974 | 0.0077519 | 0.0281124 | 0.0541455 | 0.0615079 | 0.0145530 | 0.0563725 | 0.0617978 | 0.0491228 | 0.0558376 | 0.0813953 | 0.0754717 | 0.0623145 | 0.0293542 | 0.0465839 | 0.0485774 | 0.0273462 | 0.0877193 | 0.2168803 | 0.1875729 | 0.1523605 | 0.2390110 | 0.1236133 |
| CD8 T-rm | 0.0189394 | 0.0983302 | 0.2755556 | 0.0950292 | 0.0480769 | 0.0212766 | 0.0978261 | 0.0560748 | 0.1209068 | 0.1377778 | 0.1243124 | 0.0956175 | 0.0489796 | 0.1022727 | 0.1732283 | 0.2302632 | 0.1519507 | 0.0896552 | 0.0437550 | 0.2012195 | 0.1542289 | 0.1139471 | 0.0358974 | 0.0232558 | 0.0602410 | 0.0761421 | 0.0297619 | 0.0062370 | 0.0502451 | 0.1516854 | 0.0666667 | 0.1725888 | 0.0697674 | 0.0566038 | 0.1216617 | 0.0195695 | 0.2321429 | 0.0735600 | 0.0559354 | 0.1615882 | 0.1794872 | 0.2219953 | 0.1888412 | 0.1401099 | 0.1362916 |
| cDC1 | 0.0075758 | 0.0018553 | 0.0044444 | 0.0043860 | 0.0019231 | 0.0000000 | 0.0000000 | 0.0186916 | 0.0125945 | 0.0133333 | 0.0044004 | 0.0073041 | 0.0040816 | 0.0151515 | 0.0000000 | 0.0000000 | 0.0020534 | 0.0000000 | 0.0302307 | 0.0020325 | 0.0049751 | 0.0118505 | 0.0000000 | 0.0000000 | 0.0120482 | 0.0050761 | 0.0079365 | 0.0000000 | 0.0134804 | 0.0000000 | 0.0105263 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0059347 | 0.0039139 | 0.0054348 | 0.0062457 | 0.0012430 | 0.0036934 | 0.0053419 | 0.0110852 | 0.0107296 | 0.0082418 | 0.0095087 |
| cDC2 | 0.0189394 | 0.0092764 | 0.0133333 | 0.1666667 | 0.1961538 | 0.2340426 | 0.0024155 | 0.0809969 | 0.0302267 | 0.0266667 | 0.0231023 | 0.1985392 | 0.2122449 | 0.1401515 | 0.1338583 | 0.1250000 | 0.1149897 | 0.1620690 | 0.0326173 | 0.0569106 | 0.1492537 | 0.0911577 | 0.4974359 | 0.4961240 | 0.4016064 | 0.1404399 | 0.1706349 | 0.1683992 | 0.1727941 | 0.0842697 | 0.0596491 | 0.0507614 | 0.0000000 | 0.0283019 | 0.0326409 | 0.1330724 | 0.1156832 | 0.0555170 | 0.0379117 | 0.1735919 | 0.0213675 | 0.1330222 | 0.0364807 | 0.0192308 | 0.0174326 |
| ciliated epithelial cells | 0.3371212 | 0.0389610 | 0.0000000 | 0.0146199 | 0.0538462 | 0.0425532 | 0.3019324 | 0.0903427 | 0.1209068 | 0.0933333 | 0.1903190 | 0.0152722 | 0.0081633 | 0.0151515 | 0.1968504 | 0.0164474 | 0.1334702 | 0.0206897 | 0.0023866 | 0.0081301 | 0.0000000 | 0.0373747 | 0.0461538 | 0.0542636 | 0.0361446 | 0.0338409 | 0.0674603 | 0.0353430 | 0.0122549 | 0.0842697 | 0.0245614 | 0.0456853 | 0.0232558 | 0.0188679 | 0.0771513 | 0.0215264 | 0.0240683 | 0.0111034 | 0.0242387 | 0.0313943 | 0.0016026 | 0.0137106 | 0.0128755 | 0.0137363 | 0.0063391 |
| dividing innate cells | 0.0000000 | 0.0018553 | 0.0044444 | 0.0131579 | 0.0076923 | 0.0212766 | 0.0253623 | 0.0062305 | 0.0000000 | 0.0000000 | 0.0121012 | 0.0079681 | 0.0081633 | 0.0000000 | 0.0000000 | 0.0032895 | 0.0102669 | 0.0103448 | 0.0007955 | 0.0000000 | 0.0049751 | 0.0063810 | 0.0102564 | 0.0000000 | 0.0040161 | 0.0016920 | 0.0019841 | 0.0103950 | 0.0036765 | 0.0000000 | 0.0000000 | 0.0050761 | 0.0000000 | 0.0094340 | 0.0089021 | 0.0254403 | 0.0069876 | 0.0034698 | 0.0074580 | 0.0092336 | 0.0016026 | 0.0067095 | 0.0000000 | 0.0000000 | 0.0007924 |
| gamma delta T cells | 0.0000000 | 0.0037106 | 0.0133333 | 0.0043860 | 0.0038462 | 0.0000000 | 0.0000000 | 0.0093458 | 0.0100756 | 0.0000000 | 0.0000000 | 0.0019920 | 0.0081633 | 0.0037879 | 0.0118110 | 0.0032895 | 0.0000000 | 0.0000000 | 0.0493238 | 0.0284553 | 0.0049751 | 0.0027347 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0016920 | 0.0000000 | 0.0020790 | 0.0000000 | 0.0168539 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0094340 | 0.0296736 | 0.0019569 | 0.0108696 | 0.0000000 | 0.0292107 | 0.0101570 | 0.0133547 | 0.0037923 | 0.0021459 | 0.0000000 | 0.0071315 |
| HSP+ B cells | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0019231 | 0.0000000 | 0.0000000 | 0.0031153 | 0.0000000 | 0.0000000 | 0.0429043 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0016920 | 0.0000000 | 0.0270270 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0050761 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0410959 | 0.0007764 | 0.0013879 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 |
| innate lymphocytes | 0.0265152 | 0.2374768 | 0.0755556 | 0.0423977 | 0.0500000 | 0.1276596 | 0.0205314 | 0.0467290 | 0.0176322 | 0.1155556 | 0.0275028 | 0.0285525 | 0.0775510 | 0.0643939 | 0.0433071 | 0.0164474 | 0.0266940 | 0.0241379 | 0.0747812 | 0.0569106 | 0.0099502 | 0.0282589 | 0.0051282 | 0.0077519 | 0.0281124 | 0.0304569 | 0.0416667 | 0.0041580 | 0.0612745 | 0.0224719 | 0.0315789 | 0.0253807 | 0.0116279 | 0.0094340 | 0.0356083 | 0.0078278 | 0.0194099 | 0.0263706 | 0.0391548 | 0.0618652 | 0.1778846 | 0.0361727 | 0.0515021 | 0.1043956 | 0.0174326 |
| mast cells | 0.0000000 | 0.0000000 | 0.0000000 | 0.0029240 | 0.0019231 | 0.0000000 | 0.0024155 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0110011 | 0.0006640 | 0.0163265 | 0.0113636 | 0.0078740 | 0.0000000 | 0.0205339 | 0.0000000 | 0.0015911 | 0.0101626 | 0.0000000 | 0.0218778 | 0.0000000 | 0.0000000 | 0.0080321 | 0.0033841 | 0.0039683 | 0.0062370 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0050761 | 0.0116279 | 0.0000000 | 0.0059347 | 0.0019569 | 0.0007764 | 0.0117974 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0021459 | 0.0000000 | 0.0000000 |
| migratory DC | 0.0000000 | 0.0018553 | 0.0000000 | 0.0263158 | 0.0057692 | 0.0425532 | 0.0000000 | 0.0186916 | 0.0000000 | 0.0000000 | 0.0055006 | 0.0086321 | 0.0448980 | 0.0151515 | 0.0157480 | 0.0263158 | 0.0143737 | 0.0103448 | 0.0143198 | 0.0060976 | 0.0149254 | 0.0227894 | 0.0974359 | 0.1860465 | 0.1004016 | 0.0253807 | 0.0079365 | 0.0166320 | 0.0404412 | 0.0000000 | 0.0035088 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0234834 | 0.0147516 | 0.0055517 | 0.0012430 | 0.0258541 | 0.0000000 | 0.0256709 | 0.0000000 | 0.0000000 | 0.0023772 |
| monocytes | 0.1022727 | 0.0092764 | 0.0222222 | 0.1096491 | 0.2461538 | 0.1276596 | 0.0096618 | 0.1090343 | 0.0176322 | 0.0400000 | 0.0110011 | 0.2881806 | 0.1306122 | 0.0984848 | 0.0944882 | 0.2565789 | 0.0369610 | 0.2172414 | 0.0262530 | 0.0386179 | 0.2288557 | 0.0209663 | 0.0820513 | 0.0697674 | 0.1244980 | 0.1827411 | 0.1805556 | 0.0831601 | 0.1176471 | 0.0955056 | 0.1649123 | 0.0812183 | 0.1627907 | 0.1415094 | 0.0474777 | 0.1585127 | 0.0962733 | 0.5954198 | 0.0012430 | 0.0286242 | 0.0299145 | 0.0755543 | 0.0708155 | 0.1263736 | 0.0491284 |
| neutrophil-like | 0.0000000 | 0.0000000 | 0.0000000 | 0.0307018 | 0.0153846 | 0.0000000 | 0.0000000 | 0.0031153 | 0.0000000 | 0.0000000 | 0.0121012 | 0.0039841 | 0.0040816 | 0.0113636 | 0.0000000 | 0.0098684 | 0.0205339 | 0.0068966 | 0.0023866 | 0.0060976 | 0.0099502 | 0.0446673 | 0.0256410 | 0.0542636 | 0.0361446 | 0.0186125 | 0.0059524 | 0.3409563 | 0.0159314 | 0.0056180 | 0.0070175 | 0.0152284 | 0.0000000 | 0.0094340 | 0.0000000 | 0.0645793 | 0.0372671 | 0.0027759 | 0.0174021 | 0.0101570 | 0.0021368 | 0.0020420 | 0.0000000 | 0.0000000 | 0.0000000 |
| NK cells | 0.0416667 | 0.0575139 | 0.0844444 | 0.0321637 | 0.0461538 | 0.0000000 | 0.0072464 | 0.0342679 | 0.0277078 | 0.0355556 | 0.0176018 | 0.0099602 | 0.0204082 | 0.0113636 | 0.0078740 | 0.0000000 | 0.0143737 | 0.0241379 | 0.0588703 | 0.0264228 | 0.0248756 | 0.0154968 | 0.0153846 | 0.0000000 | 0.0040161 | 0.0169205 | 0.0119048 | 0.0062370 | 0.0147059 | 0.0056180 | 0.0070175 | 0.0152284 | 0.0581395 | 0.0188679 | 0.0089021 | 0.0000000 | 0.0077640 | 0.0111034 | 0.0298322 | 0.0350877 | 0.0320513 | 0.0157526 | 0.0257511 | 0.0082418 | 0.0736926 |
| NK-T cells | 0.0000000 | 0.0278293 | 0.0044444 | 0.0029240 | 0.0019231 | 0.0000000 | 0.0036232 | 0.0062305 | 0.0075567 | 0.0044444 | 0.0022002 | 0.0000000 | 0.0040816 | 0.0113636 | 0.0000000 | 0.0032895 | 0.0041068 | 0.0034483 | 0.0031822 | 0.0000000 | 0.0000000 | 0.0027347 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0033841 | 0.0119048 | 0.0000000 | 0.0024510 | 0.0056180 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0031056 | 0.0000000 | 0.0043505 | 0.0009234 | 0.0021368 | 0.0023337 | 0.0021459 | 0.0013736 | 0.0071315 |
| plasma B cells | 0.0151515 | 0.0000000 | 0.0000000 | 0.0029240 | 0.0000000 | 0.0000000 | 0.0108696 | 0.0000000 | 0.0025189 | 0.0000000 | 0.0110011 | 0.0019920 | 0.0040816 | 0.0037879 | 0.0000000 | 0.0000000 | 0.0020534 | 0.0034483 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0027347 | 0.0000000 | 0.0000000 | 0.0040161 | 0.0016920 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0035088 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0118694 | 0.0156556 | 0.0023292 | 0.0013879 | 0.0074580 | 0.0009234 | 0.0000000 | 0.0040840 | 0.0000000 | 0.0000000 | 0.0023772 |
| plasmacytoid DC | 0.0606061 | 0.1113173 | 0.0444444 | 0.0160819 | 0.0153846 | 0.0212766 | 0.0024155 | 0.0467290 | 0.0680101 | 0.0088889 | 0.0374037 | 0.0305445 | 0.0081633 | 0.0075758 | 0.0078740 | 0.0032895 | 0.0102669 | 0.0655172 | 0.0326173 | 0.0040650 | 0.0099502 | 0.0145852 | 0.0102564 | 0.0000000 | 0.0321285 | 0.0135364 | 0.0773810 | 0.0311850 | 0.0147059 | 0.0337079 | 0.0350877 | 0.0101523 | 0.0465116 | 0.0471698 | 0.0237389 | 0.0078278 | 0.0046584 | 0.0034698 | 0.0000000 | 0.0203139 | 0.0048077 | 0.0093349 | 0.0300429 | 0.0384615 | 0.0150555 |
| proliferating T/NK | 0.0189394 | 0.0166976 | 0.0133333 | 0.0073099 | 0.0096154 | 0.0000000 | 0.0120773 | 0.0186916 | 0.0226700 | 0.0311111 | 0.0011001 | 0.0053121 | 0.0040816 | 0.0000000 | 0.0000000 | 0.0164474 | 0.0000000 | 0.0068966 | 0.0190931 | 0.0040650 | 0.0099502 | 0.0036463 | 0.0000000 | 0.0077519 | 0.0080321 | 0.0084602 | 0.0138889 | 0.0020790 | 0.0036765 | 0.0112360 | 0.0140351 | 0.0101523 | 0.0232558 | 0.0283019 | 0.0059347 | 0.0019569 | 0.0116460 | 0.0041638 | 0.0037290 | 0.0036934 | 0.0197650 | 0.0020420 | 0.0150215 | 0.0123626 | 0.0126783 |
| secretory epithelial cells | 0.0454545 | 0.0500928 | 0.0088889 | 0.0029240 | 0.0134615 | 0.0638298 | 0.1159420 | 0.0155763 | 0.0226700 | 0.0222222 | 0.0440044 | 0.0059761 | 0.0000000 | 0.0113636 | 0.0708661 | 0.0000000 | 0.0102669 | 0.0068966 | 0.0000000 | 0.0000000 | 0.0000000 | 0.0054695 | 0.0256410 | 0.0387597 | 0.0281124 | 0.0456853 | 0.0317460 | 0.0124740 | 0.0049020 | 0.0393258 | 0.0105263 | 0.0203046 | 0.0465116 | 0.0377358 | 0.0415430 | 0.0000000 | 0.0038820 | 0.0069396 | 0.0385333 | 0.0424746 | 0.0389957 | 0.0107935 | 0.0107296 | 0.0041209 | 0.0023772 |
Cell type proportions by sample
Create sample meta data table.
seu@meta.data %>%
dplyr::select(sample.id,
Participant,
Disease,
Treatment,
Severity,
Group,
Group_severity,
Batch,
Age,
Sex) %>%
left_join(props$Counts %>%
data.frame %>%
group_by(sample) %>%
summarise(ncells = sum(Freq)),
by = c("sample.id" = "sample")) %>%
distinct() -> info
head(info) %>% knitr::kable()| sample.id | Participant | Disease | Treatment | Severity | Group | Group_severity | Batch | Age | Sex | ncells |
|---|---|---|---|---|---|---|---|---|---|---|
| sample_33.1 | sample_33 | CF | treated (ivacaftor) | severe | CF.IVA | CF.IVA.S | 1 | 5.950685 | M | 1097 |
| sample_25.1 | sample_25 | CF | untreated | severe | CF.NO_MOD | CF.NO_MOD.S | 1 | 4.910000 | F | 245 |
| sample_29.1 | sample_29 | CF | untreated | severe | CF.NO_MOD | CF.NO_MOD.S | 1 | 5.989041 | F | 487 |
| sample_27.1 | sample_27 | CF | treated (ivacaftor) | mild | CF.IVA | CF.IVA.M | 1 | 4.917808 | M | 254 |
| sample_32.1 | sample_32 | CF | untreated | mild | CF.NO_MOD | CF.NO_MOD.M | 1 | 5.926027 | F | 201 |
| sample_26.1 | sample_26 | CF | untreated | mild | CF.NO_MOD | CF.NO_MOD.M | 1 | 5.049315 | M | 264 |
props$Proportions %>%
data.frame %>%
left_join(info,
by = c("sample" = "sample.id")) %>%
ggplot(aes(x = sample, y = Freq, fill = clusters)) +
geom_bar(stat = "identity", color = "black", size = 0.1) +
theme(axis.text.x = element_text(angle = 90,
vjust = 0.5,
hjust = 1),
legend.text = element_text(size = 8),
legend.position = "bottom") +
labs(y = "Proportion", fill = "Cell Label") +
facet_grid(~Group, scales = "free_x", space = "free_x")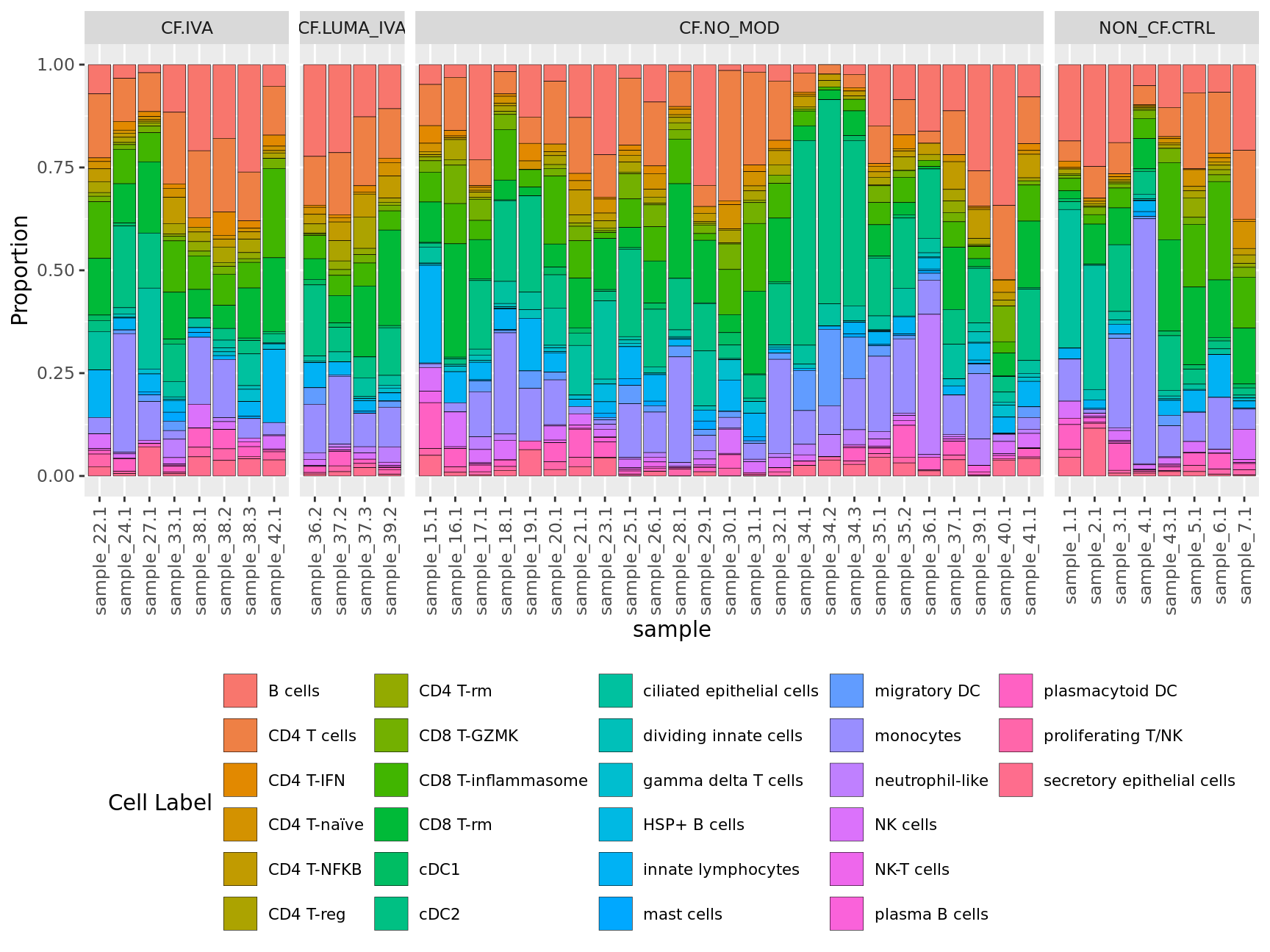
No. cells per sample
info %>%
ggplot(aes(x = sample.id, y = ncells, fill = Disease)) +
geom_bar(stat = "identity") +
theme(axis.text.x = element_text(angle = 90,
vjust = 0.5,
hjust = 1),
legend.text = element_text(size = 8),
legend.position = "bottom") +
labs(y = "No. cells", fill = "Disease") +
facet_grid(~Group, scales = "free_x", space = "free_x") +
geom_hline(yintercept = 100, linetype = "dashed")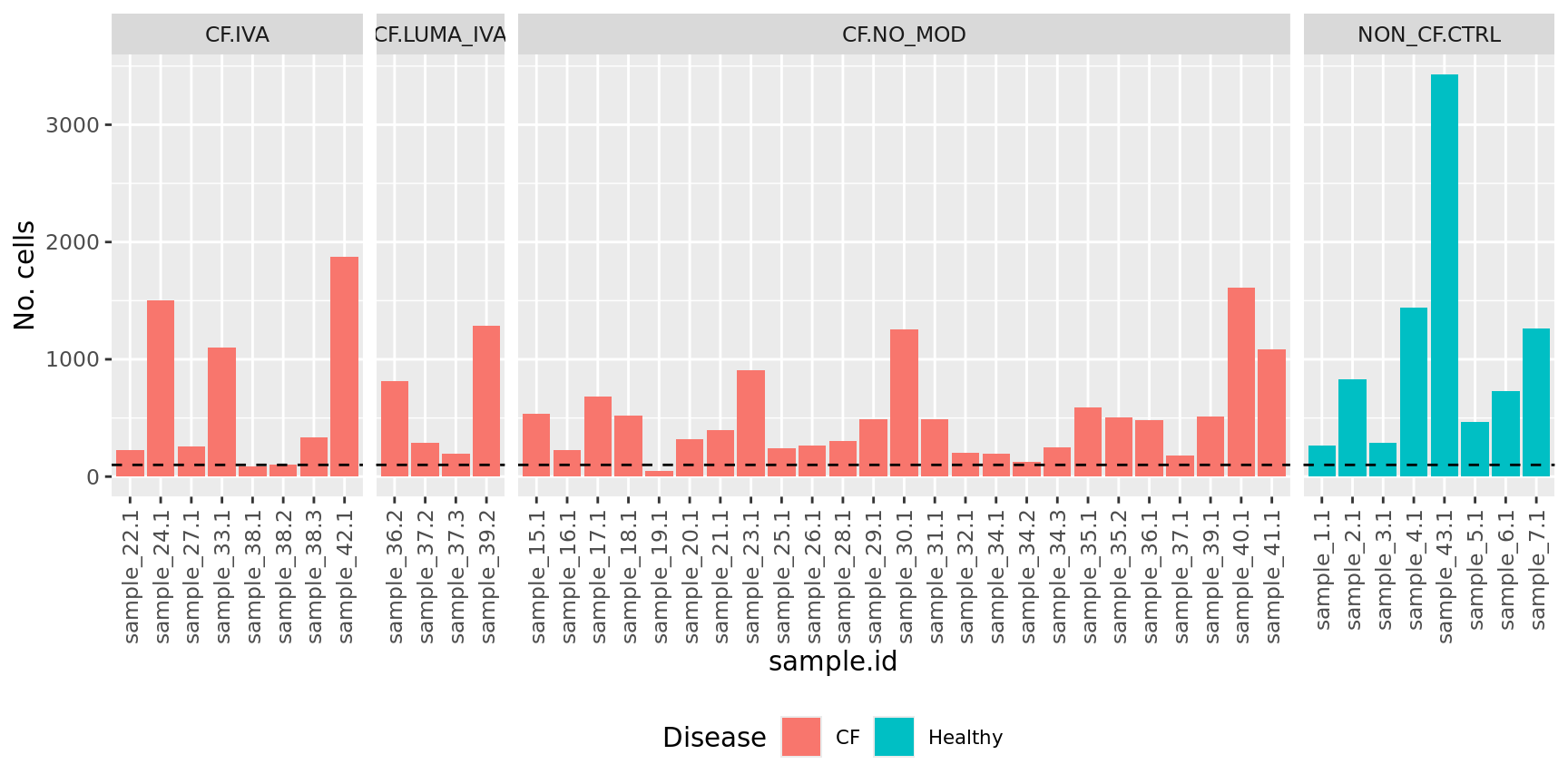
Cell proportions by cell type
props$Proportions %>%
data.frame %>%
left_join(info,
by = c("sample" = "sample.id")) %>%
ggplot(aes(x = clusters, y = Freq, fill = clusters)) +
geom_boxplot(outlier.size = 0.1, size = 0.25) +
theme(axis.text.x = element_text(angle = 45,
vjust = 1,
hjust = 1),
legend.text = element_text(size = 8)) +
labs(y = "Proportion") +
NoLegend()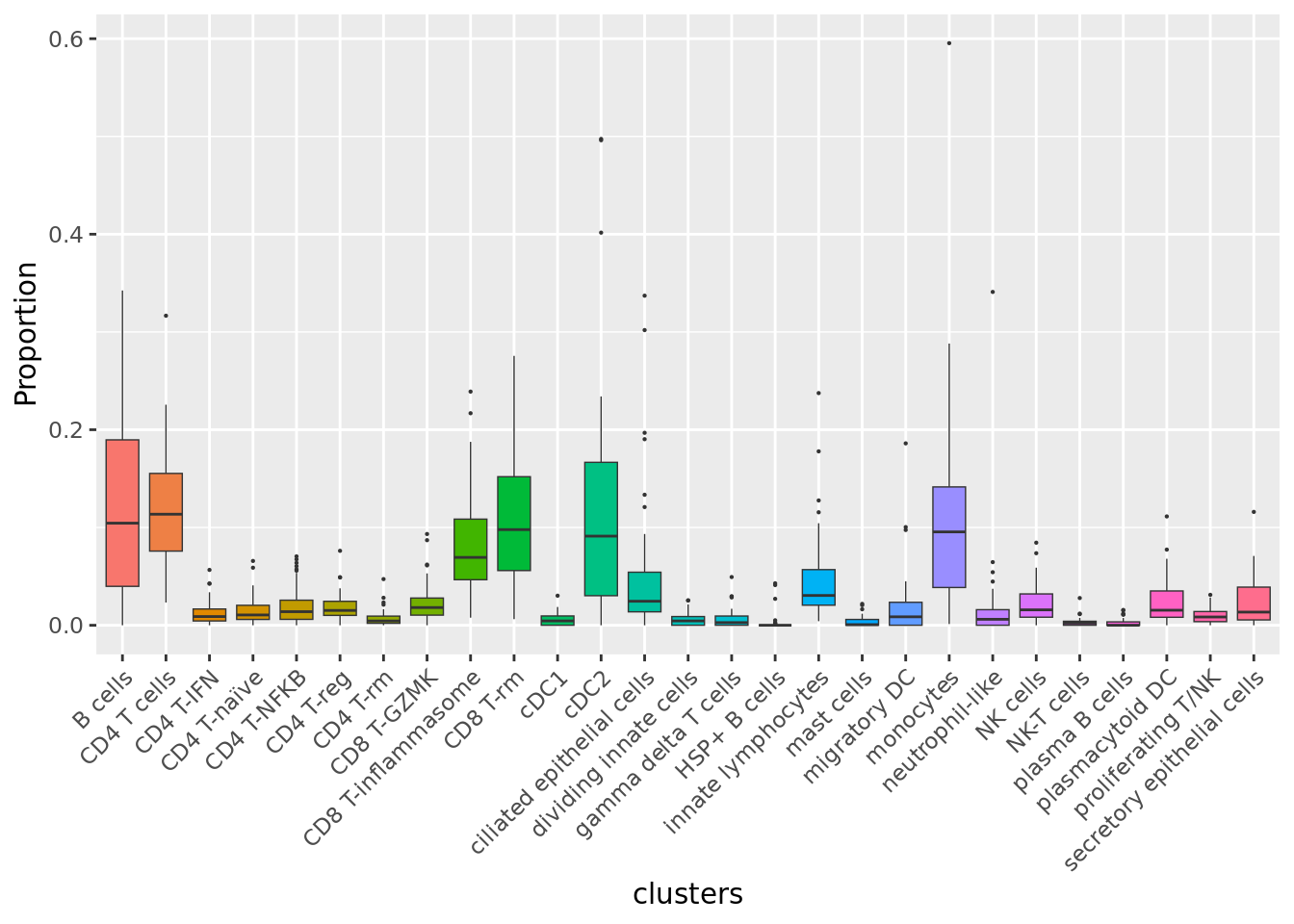
Explore sources of variation
Cell count data
Look at the sources of variation in the raw cell count level data.
dims <- list(c(1,2), c(2:3), c(3,4), c(4,5))
p <- vector("list", length(dims))
for(i in 1:length(dims)){
mds <- plotMDS(props$Counts,
gene.selection = "common",
plot = FALSE, dim.plot = dims[[i]])
data.frame(x = mds$x,
y = mds$y,
sample = rownames(mds$distance.matrix.squared)) %>%
left_join(info,
by = c("sample" = "sample.id")) %>%
distinct() -> dat
p[[i]] <- ggplot(dat, aes(x = x, y = y,
shape = as.factor(Disease),
color = as.factor(Batch))) +
geom_point(size = 3) +
labs(x = glue("Principal Component {dims[[i]][1]}"),
y = glue("Principal Component {dims[[i]][2]}"),
colour = "Batch",
shape = "Disease") +
theme(legend.direction = "horizontal",
legend.text = element_text(size = 8),
legend.title = element_text(size = 9),
axis.text = element_text(size = 8),
axis.title = element_text(size = 9))
}
wrap_plots(p, cols = 2) + plot_layout(guides = "collect") &
theme(legend.position = "bottom") 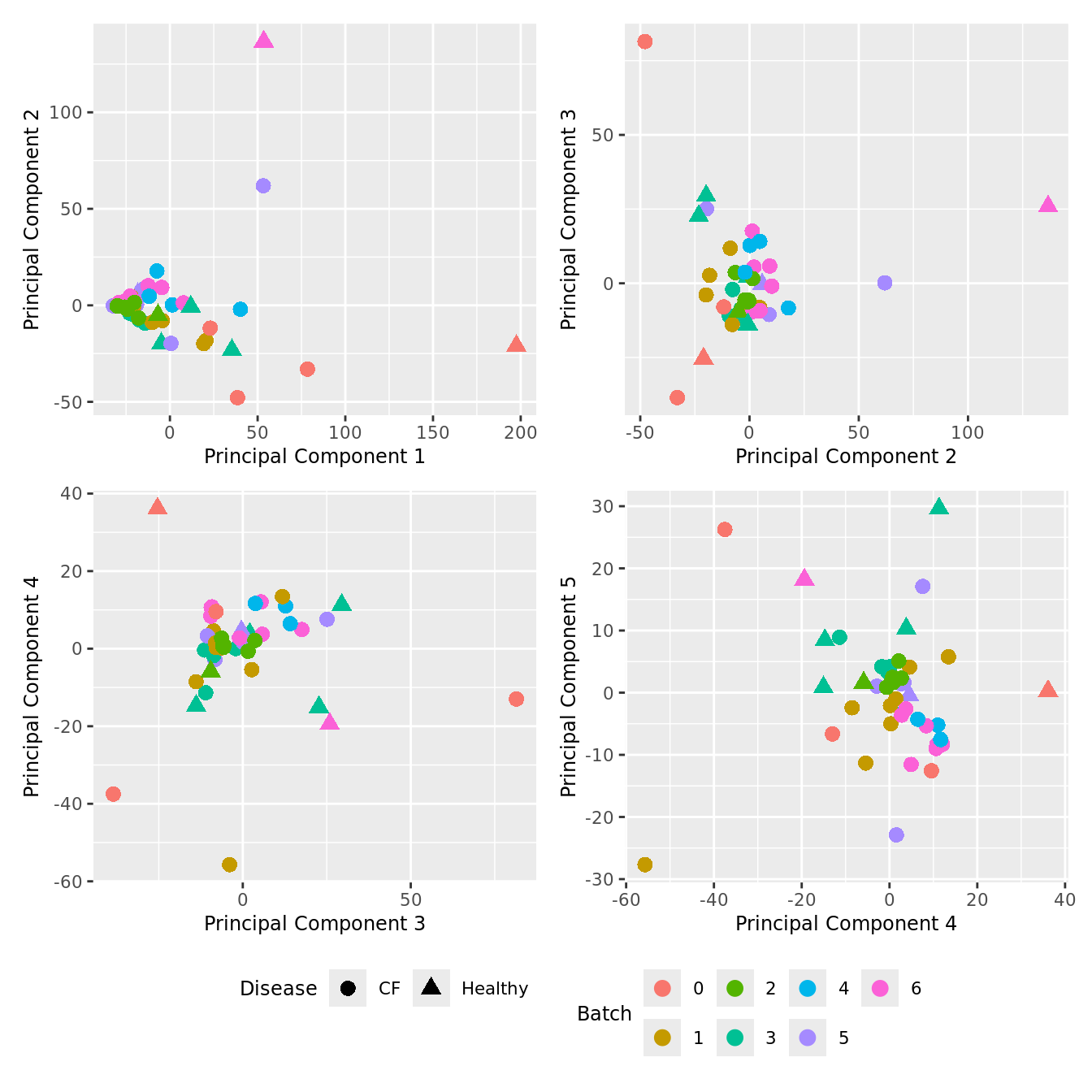
dims <- list(c(1,2), c(2:3), c(3,4), c(4,5))
p <- vector("list", length(dims))
for(i in 1:length(dims)){
mds <- plotMDS(props$Counts,
gene.selection = "common",
plot = FALSE, dim.plot = dims[[i]])
data.frame(x = mds$x,
y = mds$y,
sample = rownames(mds$distance.matrix.squared)) %>%
left_join(info,
by = c("sample" = "sample.id")) %>%
distinct() -> dat
p[[i]] <- ggplot(dat, aes(x = x, y = y,
colour = log2(ncells)))+
geom_text(aes(label = str_remove_all(sample, "sample_")), size = 2.5) +
labs(x = glue("Principal Component {dims[[i]][1]}"),
y = glue("Principal Component {dims[[i]][2]}"),
colour = "Log2 No. Cells") +
theme(legend.direction = "horizontal",
legend.text = element_text(size = 8),
legend.title = element_text(size = 9),
axis.text = element_text(size = 8),
axis.title = element_text(size = 9)) +
scale_colour_viridis_c(option = "magma")
}
wrap_plots(p, cols = 2) + plot_layout(guides = "collect") &
theme(legend.position = "bottom") 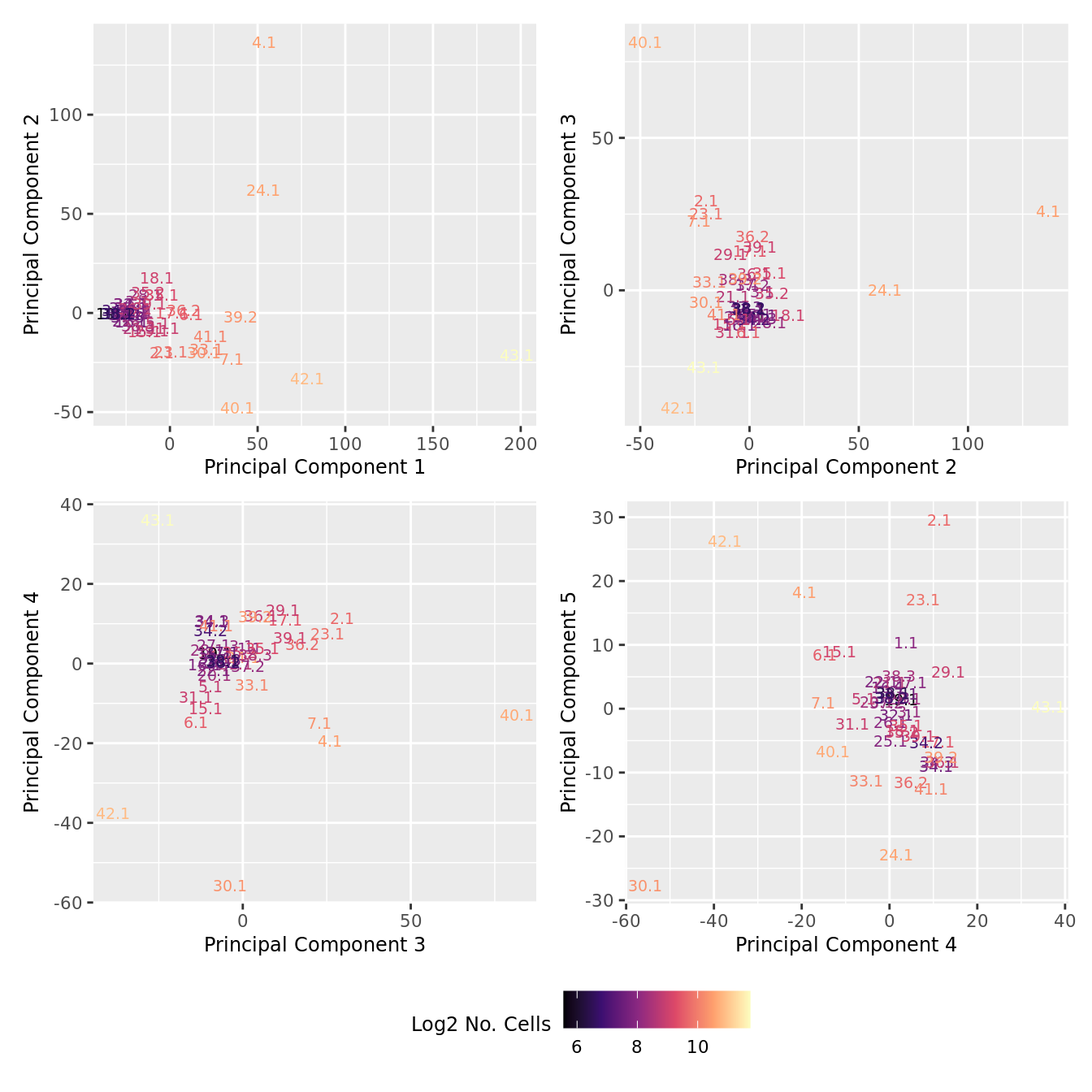
Cell proportion data
Look at the sources of variation in the cell proportions data.
dims <- list(c(1,2), c(2:3), c(3,4), c(4,5))
p <- vector("list", length(dims))
for(i in 1:length(dims)){
mds <- plotMDS(props$TransformedProps,
gene.selection = "common",
plot = FALSE, dim.plot = dims[[i]])
data.frame(x = mds$x,
y = mds$y,
sample = rownames(mds$distance.matrix.squared)) %>%
left_join(info,
by = c("sample" = "sample.id")) %>%
distinct() -> dat
p[[i]] <- ggplot(dat, aes(x = x, y = y,
shape = as.factor(Disease),
color = as.factor(Batch)))+
geom_point(size = 3) +
labs(x = glue("Principal Component {dims[[i]][1]}"),
y = glue("Principal Component {dims[[i]][2]}"),
colour = "Batch",
shape = "Disease") +
theme(legend.direction = "horizontal",
legend.text = element_text(size = 8),
legend.title = element_text(size = 9),
axis.text = element_text(size = 8),
axis.title = element_text(size = 9))
}
wrap_plots(p, cols = 2) + plot_layout(guides = "collect") &
theme(legend.position = "bottom") 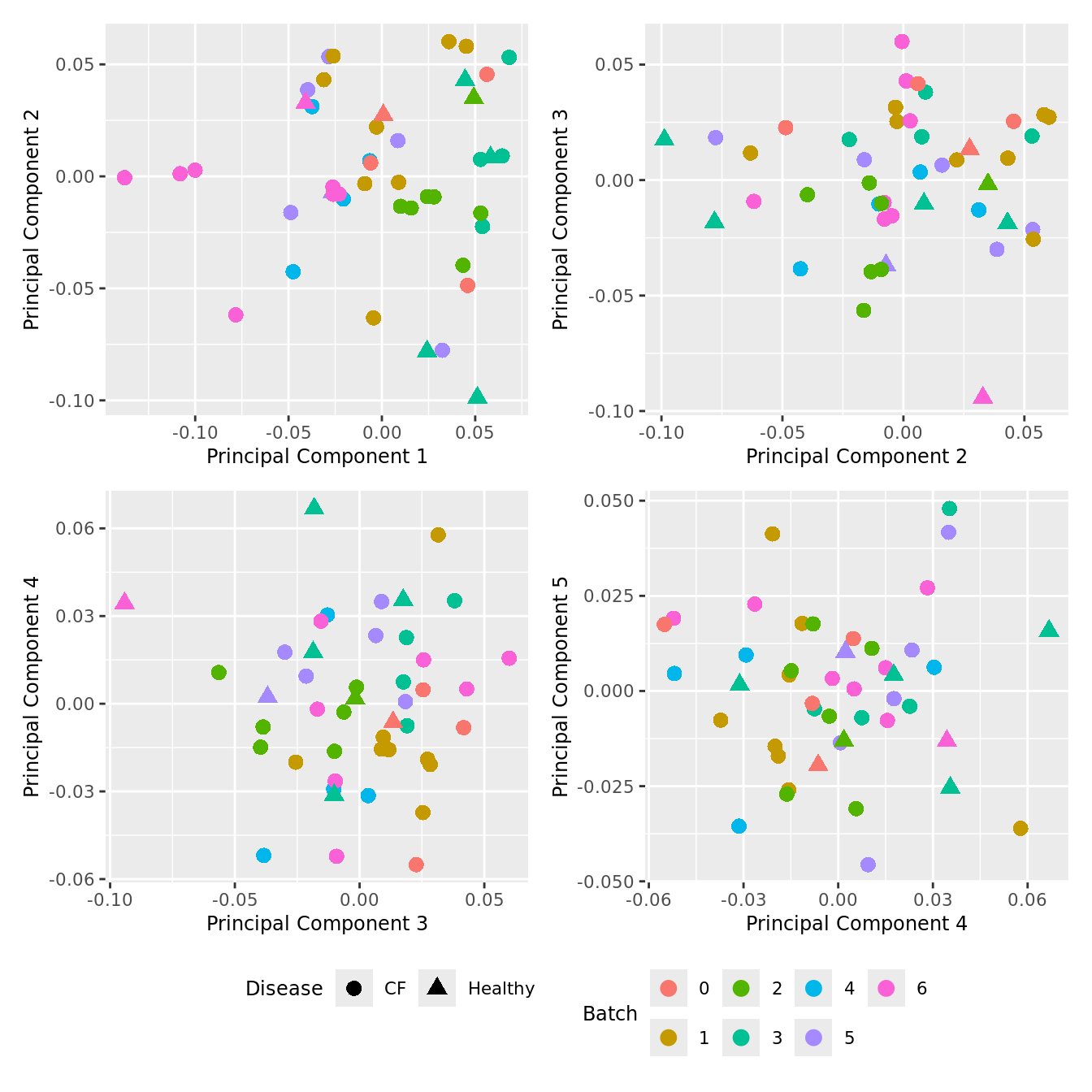
dims <- list(c(1,2), c(2:3), c(3,4), c(4,5))
p <- vector("list", length(dims))
for(i in 1:length(dims)){
mds <- plotMDS(props$TransformedProps,
gene.selection = "common",
plot = FALSE, dim.plot = dims[[i]])
data.frame(x = mds$x,
y = mds$y,
sample = rownames(mds$distance.matrix.squared)) %>%
left_join(info,
by = c("sample" = "sample.id")) %>%
distinct() -> dat
p[[i]] <- ggplot(dat, aes(x = x, y = y,
shape = as.factor(Disease),
color = Sex))+
geom_point(size = 3) +
labs(x = glue("Principal Component {dims[[i]][1]}"),
y = glue("Principal Component {dims[[i]][2]}"),
colour = "Sex",
shape = "Disease") +
theme(legend.direction = "horizontal",
legend.text = element_text(size = 8),
legend.title = element_text(size = 9),
axis.text = element_text(size = 8),
axis.title = element_text(size = 9))
}
wrap_plots(p, cols = 2) + plot_layout(guides = "collect") &
theme(legend.position = "bottom") 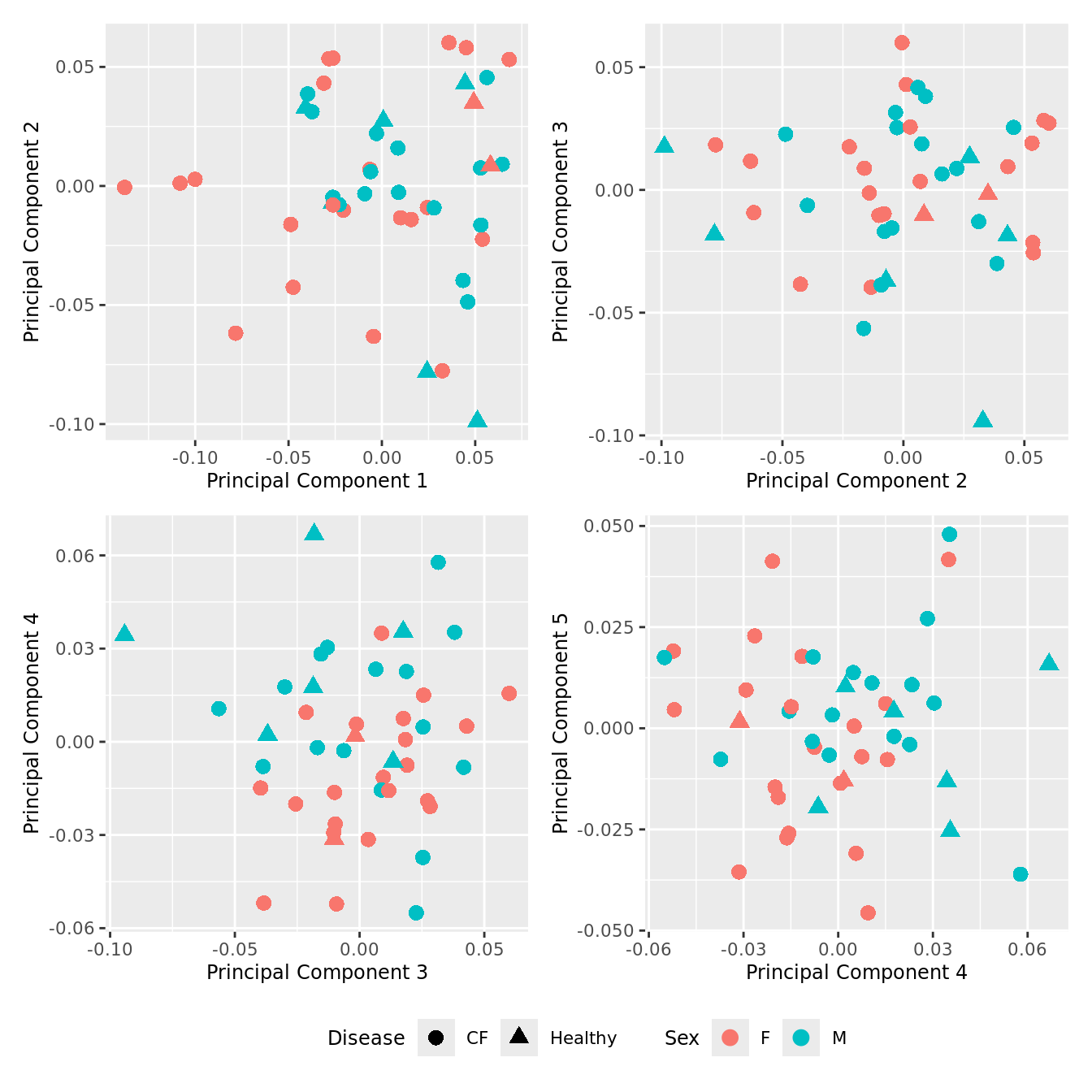
dims <- list(c(1,2), c(2:3), c(3,4), c(4,5))
p <- vector("list", length(dims))
for(i in 1:length(dims)){
mds <- plotMDS(props$TransformedProps,
gene.selection = "common",
plot = FALSE, dim.plot = dims[[i]])
data.frame(x = mds$x,
y = mds$y,
sample = rownames(mds$distance.matrix.squared)) %>%
left_join(info,
by = c("sample" = "sample.id")) %>%
distinct() -> dat
p[[i]] <- ggplot(dat, aes(x = x, y = y,
colour = log2(Age)))+
geom_text(aes(label = str_remove_all(sample, "sample_")), size = 2.5) +
labs(x = glue("Principal Component {dims[[i]][1]}"),
y = glue("Principal Component {dims[[i]][2]}"),
colour = "Log2 Age") +
theme(legend.direction = "horizontal",
legend.text = element_text(size = 8),
legend.title = element_text(size = 9),
axis.text = element_text(size = 8),
axis.title = element_text(size = 9)) +
scale_colour_viridis_c(option = "magma")
}
wrap_plots(p, cols = 2) + plot_layout(guides = "collect") &
theme(legend.position = "bottom") 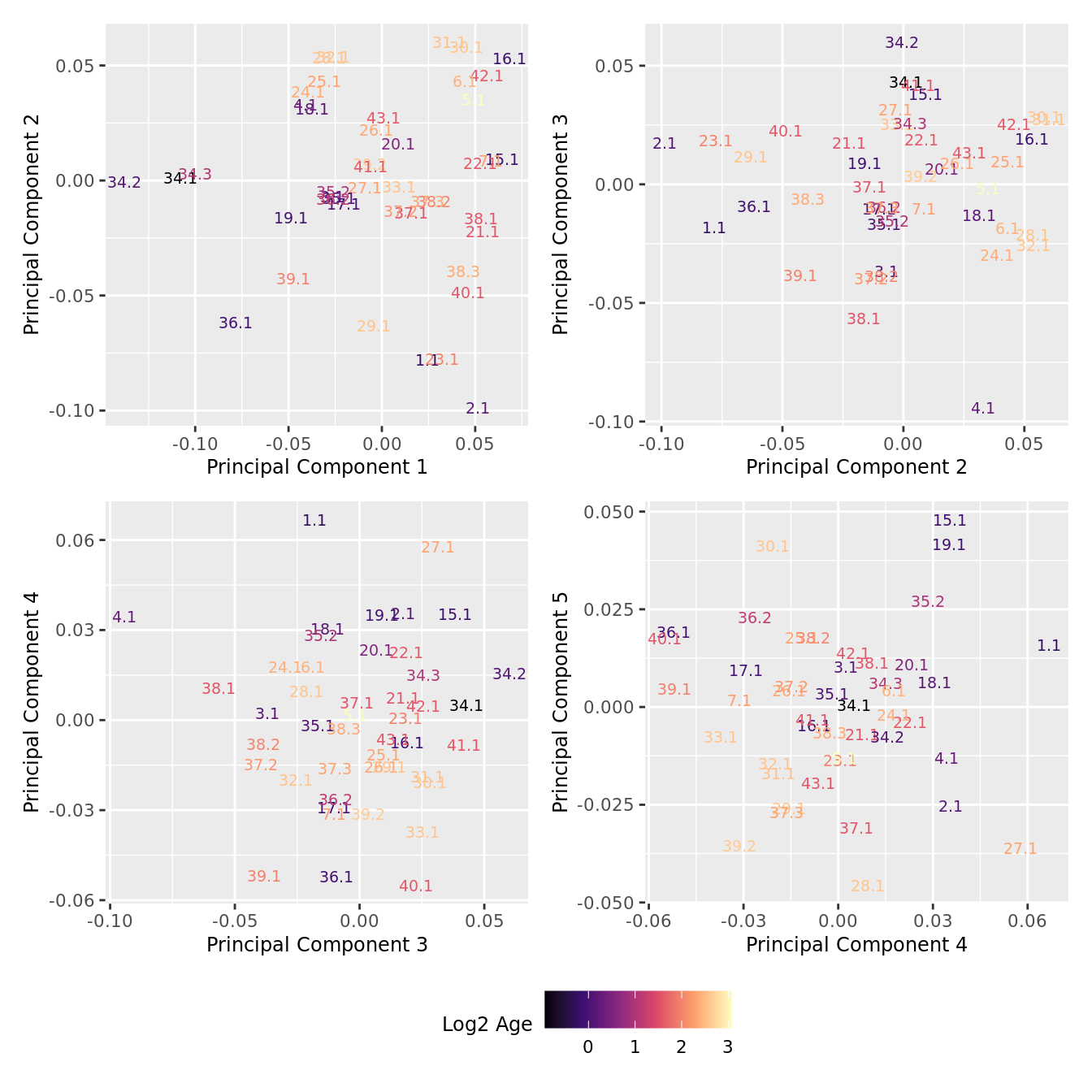
dims <- list(c(1,2), c(2:3), c(3,4), c(4,5))
p <- vector("list", length(dims))
for(i in 1:length(dims)){
mds <- plotMDS(props$TransformedProps,
gene.selection = "common",
plot = FALSE, dim.plot = dims[[i]])
data.frame(x = mds$x,
y = mds$y,
sample = rownames(mds$distance.matrix.squared)) %>%
left_join(info,
by = c("sample" = "sample.id")) %>%
distinct() -> dat
p[[i]] <- ggplot(dat, aes(x = x, y = y,
colour = log2(ncells)))+
geom_text(aes(label = str_remove_all(sample, "sample_")), size = 2.5) +
labs(x = glue("Principal Component {dims[[i]][1]}"),
y = glue("Principal Component {dims[[i]][2]}"),
colour = "Log2 No. Cells") +
theme(legend.direction = "horizontal",
legend.text = element_text(size = 8),
legend.title = element_text(size = 9),
axis.text = element_text(size = 8),
axis.title = element_text(size = 9)) +
scale_colour_viridis_c(option = "magma")
}
wrap_plots(p, cols = 2) + plot_layout(guides = "collect") &
theme(legend.position = "bottom") 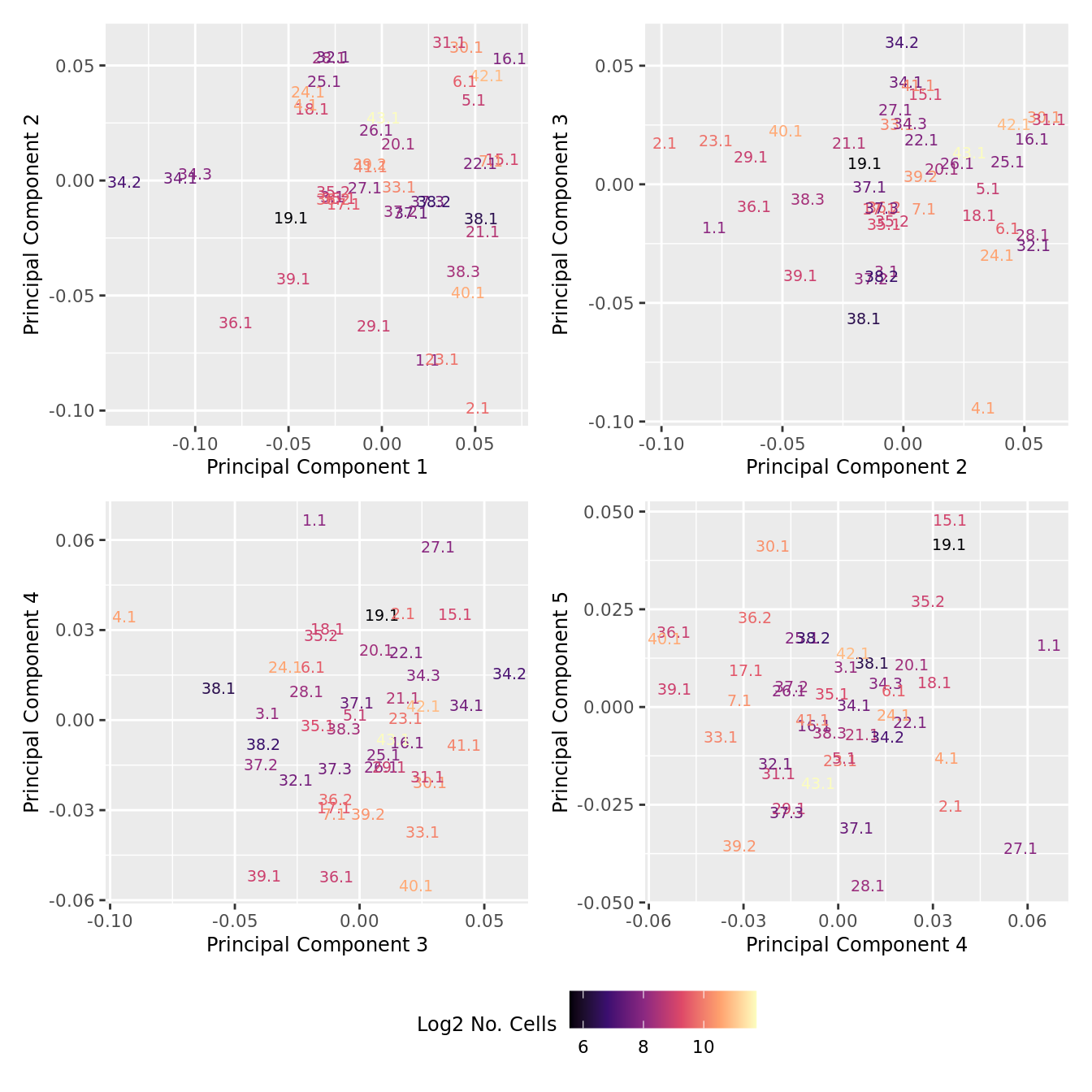
Principal components versus traits
Principal components analysis (PCA) allows us to mathematically determine the sources of variation in the data. We can then investigate whether these correlate with any of the specifed covariates. First, we calculate the principal components. The scree plot belows shows us that most of the variation in this data is captured by the top 7 principal components.
PCs <- prcomp(t(props$TransformedProps), center = TRUE,
scale = TRUE, retx = TRUE)
loadings = PCs$x # pc loadings
plot(PCs, type="lines") # scree plot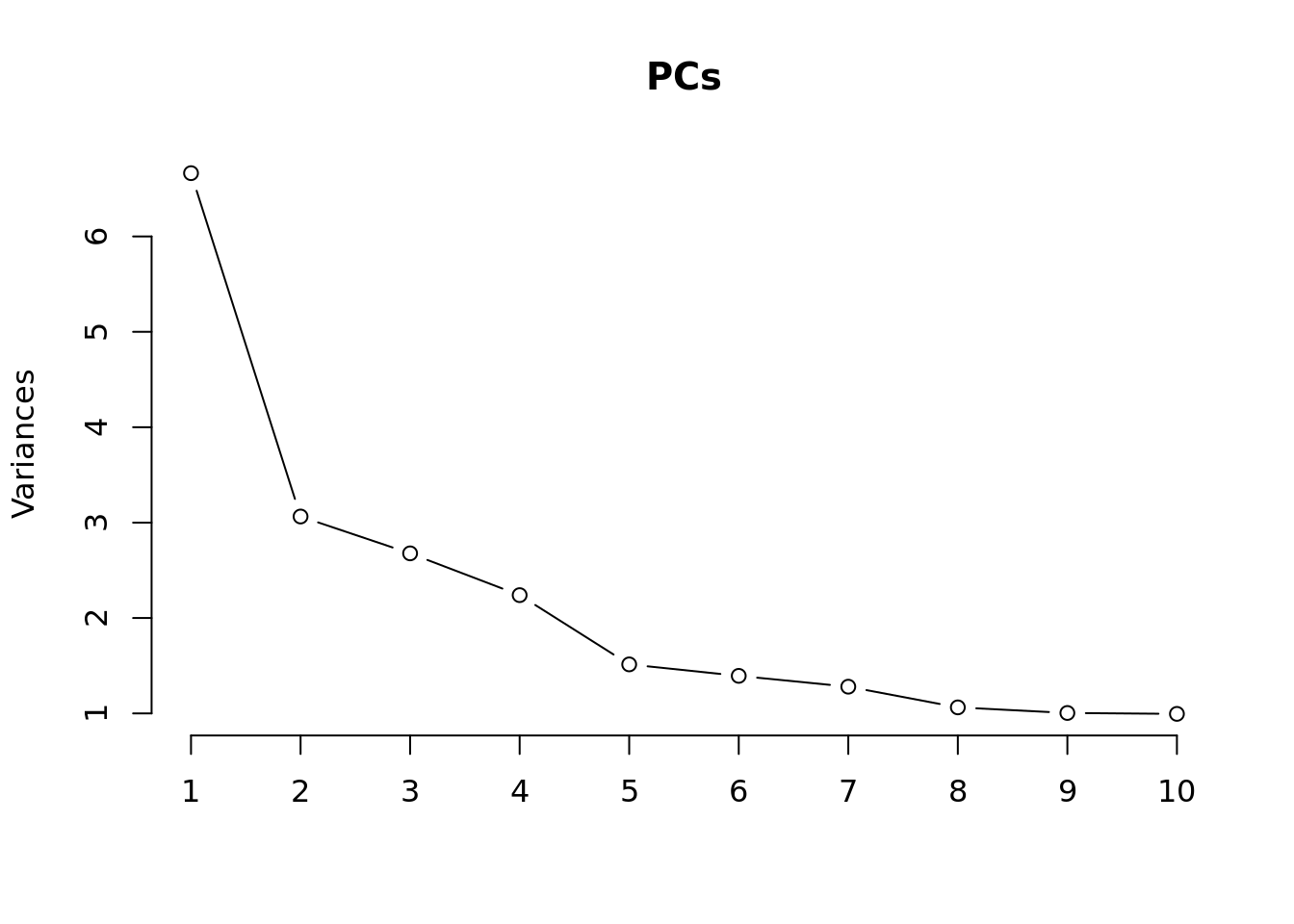
Collect all of the known sample traits.
nGenes = nrow(props$TransformedProps)
nSamples = ncol(props$TransformedProps)
m <- match(colnames(props$TransformedProps), info$sample.id)
info <- info[m,]
datTraits <- info %>% dplyr::select(Participant, Batch, Disease, Treatment,
Group, Severity, Age, Sex, ncells) %>%
mutate(Age = log2(Age),
ncells = log2(ncells),
Donor = factor(Participant),
Batch = factor(Batch),
Disease = factor(Disease,
labels = 1:length(unique(Disease))),
Group = factor(Group,
labels = 1:length(unique(Group))),
Treatment = factor(Treatment,
labels = 1:length(unique(Treatment))),
Sex = factor(Sex, labels = length(unique(Sex))),
Severity = factor(Severity, labels = length(unique(Severity)))) %>%
mutate(across(everything(), as.numeric)) %>%
dplyr::select(-Participant)
datTraits %>%
knitr::kable()| Batch | Disease | Treatment | Group | Severity | Age | Sex | ncells | Donor | |
|---|---|---|---|---|---|---|---|---|---|
| 19 | 4 | 2 | 1 | 4 | 1 | -0.2590872 | 2 | 8.044394 | 1 |
| 18 | 4 | 1 | 4 | 3 | 2 | -0.0939001 | 2 | 9.074141 | 2 |
| 16 | 4 | 1 | 4 | 3 | 2 | -0.1151479 | 1 | 7.813781 | 3 |
| 24 | 5 | 1 | 4 | 3 | 2 | -0.0441471 | 1 | 9.417853 | 4 |
| 27 | 5 | 1 | 4 | 3 | 2 | 0.1428834 | 2 | 9.022368 | 5 |
| 33 | 6 | 1 | 4 | 3 | 2 | -0.0729608 | 1 | 5.554589 | 6 |
| 17 | 4 | 2 | 1 | 4 | 1 | 0.1464588 | 2 | 9.693487 | 7 |
| 32 | 6 | 1 | 4 | 3 | 3 | 0.5597097 | 2 | 8.326429 | 8 |
| 22 | 4 | 1 | 4 | 3 | 3 | 1.5743836 | 1 | 8.632995 | 9 |
| 23 | 4 | 1 | 2 | 1 | 2 | 1.5993830 | 2 | 7.813781 | 10 |
| 31 | 6 | 1 | 4 | 3 | 3 | 1.9720631 | 1 | 9.828136 | 11 |
| 28 | 6 | 1 | 2 | 1 | 2 | 2.3883594 | 2 | 10.556506 | 12 |
| 2 | 2 | 1 | 4 | 3 | 3 | 2.2957230 | 1 | 7.936638 | 13 |
| 6 | 2 | 1 | 4 | 3 | 2 | 2.3360877 | 2 | 8.044394 | 14 |
| 4 | 2 | 1 | 2 | 1 | 2 | 2.2980155 | 2 | 7.988685 | 15 |
| 29 | 6 | 1 | 4 | 3 | 2 | 2.5790214 | 1 | 8.247927 | 16 |
| 3 | 2 | 1 | 4 | 3 | 3 | 2.5823250 | 1 | 8.927778 | 17 |
| 30 | 6 | 2 | 1 | 4 | 1 | 0.1321035 | 2 | 8.179909 | 18 |
| 7 | 2 | 1 | 4 | 3 | 3 | 2.5889097 | 1 | 10.295769 | 19 |
| 8 | 2 | 1 | 4 | 3 | 2 | 2.5583683 | 1 | 8.942514 | 20 |
| 5 | 2 | 1 | 4 | 3 | 2 | 2.5670653 | 1 | 7.651052 | 21 |
| 1 | 2 | 1 | 2 | 1 | 3 | 2.5730557 | 2 | 10.099348 | 22 |
| 40 | 7 | 1 | 4 | 3 | 2 | -0.9343238 | 1 | 7.607330 | 23 |
| 41 | 7 | 1 | 4 | 3 | 2 | 0.0918737 | 1 | 7.011227 | 23 |
| 34 | 7 | 1 | 4 | 3 | 2 | 1.0409164 | 1 | 7.960002 | 23 |
| 35 | 7 | 1 | 4 | 3 | 2 | 0.0807044 | 2 | 9.207014 | 24 |
| 39 | 7 | 1 | 4 | 3 | 2 | 0.9940589 | 2 | 8.977280 | 24 |
| 38 | 7 | 1 | 4 | 3 | 3 | -0.0564254 | 1 | 8.909893 | 25 |
| 37 | 7 | 1 | 3 | 2 | 3 | 1.1764977 | 1 | 9.672425 | 25 |
| 10 | 3 | 1 | 4 | 3 | 2 | 1.5597097 | 1 | 7.475733 | 26 |
| 9 | 3 | 1 | 3 | 2 | 2 | 2.1930156 | 1 | 8.154818 | 26 |
| 11 | 3 | 1 | 3 | 2 | 2 | 2.2980155 | 1 | 7.622052 | 26 |
| 14 | 3 | 1 | 2 | 1 | 2 | 1.5703964 | 2 | 6.426265 | 27 |
| 15 | 3 | 1 | 2 | 1 | 2 | 2.0206033 | 2 | 6.727920 | 27 |
| 13 | 3 | 1 | 2 | 1 | 2 | 2.3485584 | 2 | 8.396605 | 27 |
| 26 | 5 | 1 | 4 | 3 | 2 | 1.9730702 | 1 | 8.997179 | 28 |
| 25 | 5 | 1 | 3 | 2 | 2 | 2.6297159 | 1 | 10.330917 | 28 |
| 36 | 7 | 2 | 1 | 4 | 1 | 0.2923784 | 2 | 10.492855 | 29 |
| 42 | 1 | 1 | 4 | 3 | 3 | 1.5801455 | 2 | 10.651949 | 30 |
| 43 | 1 | 1 | 4 | 3 | 2 | 1.5801455 | 2 | 10.080818 | 31 |
| 45 | 1 | 1 | 2 | 1 | 3 | 1.5993178 | 2 | 10.870365 | 32 |
| 44 | 1 | 2 | 1 | 4 | 1 | 1.5849625 | 2 | 11.743151 | 33 |
| 12 | 3 | 2 | 1 | 4 | 1 | 3.0699187 | 1 | 8.864186 | 34 |
| 20 | 4 | 2 | 1 | 4 | 1 | 2.4204621 | 2 | 9.507795 | 35 |
| 21 | 4 | 2 | 1 | 4 | 1 | 2.2356012 | 1 | 10.301496 | 36 |
Correlate known sample traits with the top 10 principal components. This can help us determine which traits are potentially contributing to the main sources of variation in the data and should thus be included in our statistical analysis.
moduleTraitCor <- suppressWarnings(cor(loadings[, 1:10], datTraits, use = "p"))
moduleTraitPvalue <- WGCNA::corPvalueStudent(moduleTraitCor, (nSamples - 2))
textMatrix <- paste(signif(moduleTraitCor, 2), "\n(",
signif(moduleTraitPvalue, 1), ")", sep = "")
dim(textMatrix) <- dim(moduleTraitCor)
## Display the correlation values within a heatmap plot
par(cex=0.75, mar = c(6, 8.5, 3, 3))
WGCNA::labeledHeatmap(Matrix = t(moduleTraitCor),
xLabels = colnames(loadings)[1:10],
yLabels = names(datTraits),
colorLabels = FALSE,
colors = WGCNA::blueWhiteRed(6),
textMatrix = t(textMatrix),
setStdMargins = FALSE,
cex.text = 1,
zlim = c(-1,1),
main = paste("PCA-trait relationships: Top 10 PCs"))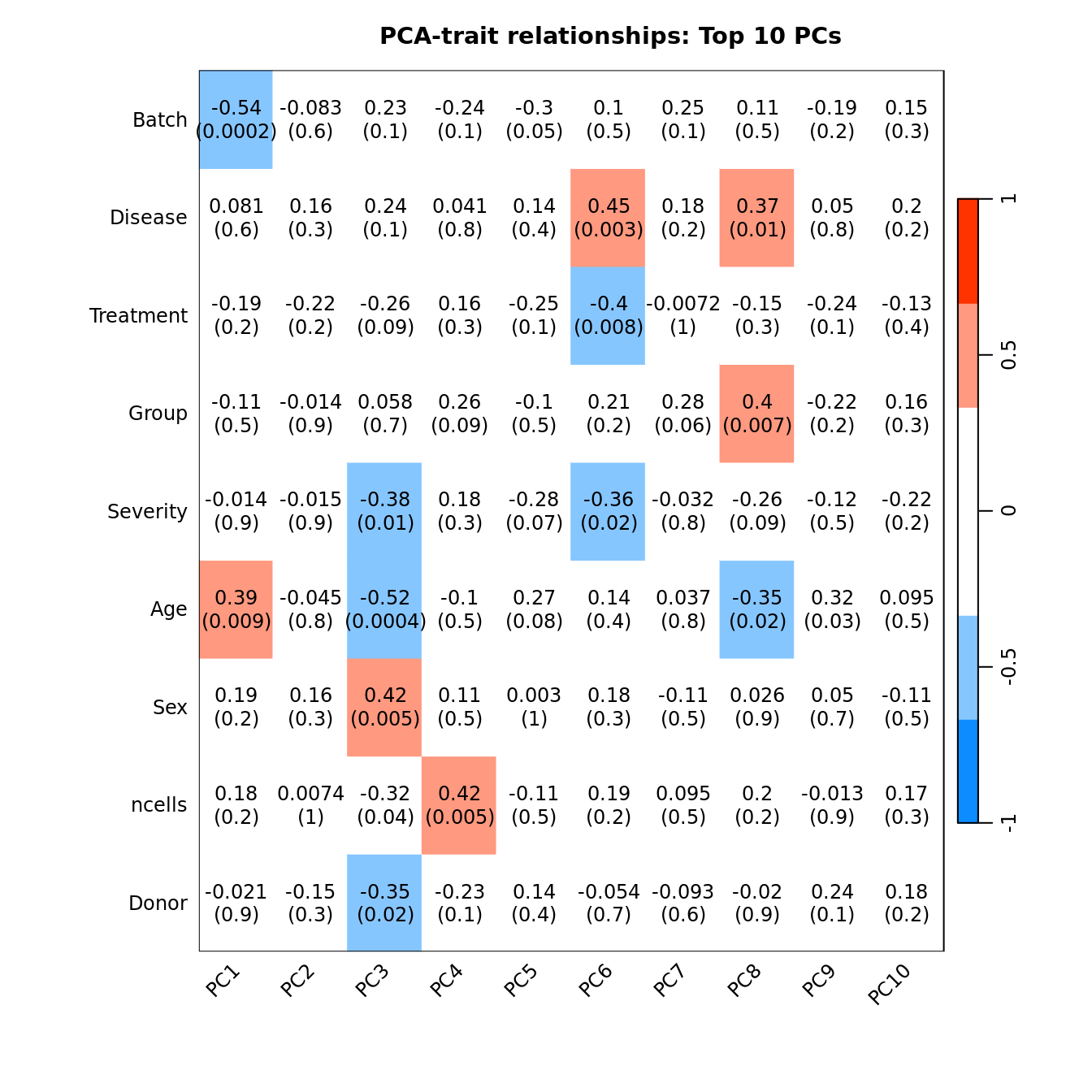
Statistical analysis using propeller and
limma
Create the design matrix.
group <- factor(info$Group_severity)
participant <- factor(info$Participant)
age <- log2(info$Age)
batch <- factor(info$Batch)
sex <- factor(info$Sex)
design <- model.matrix(~ 0 + group + batch + age + sex)
colnames(design)[1:7] <- levels(group)
design CF.IVA.M CF.IVA.S CF.LUMA_IVA.M CF.LUMA_IVA.S CF.NO_MOD.M CF.NO_MOD.S
1 0 0 0 0 0 0
2 0 0 0 0 1 0
3 0 0 0 0 1 0
4 0 0 0 0 1 0
5 0 0 0 0 1 0
6 0 0 0 0 1 0
7 0 0 0 0 0 0
8 0 0 0 0 0 1
9 0 0 0 0 0 1
10 1 0 0 0 0 0
11 0 0 0 0 0 1
12 1 0 0 0 0 0
13 0 0 0 0 0 1
14 0 0 0 0 1 0
15 1 0 0 0 0 0
16 0 0 0 0 1 0
17 0 0 0 0 0 1
18 0 0 0 0 0 0
19 0 0 0 0 0 1
20 0 0 0 0 1 0
21 0 0 0 0 1 0
22 0 1 0 0 0 0
23 0 0 0 0 1 0
24 0 0 0 0 1 0
25 0 0 0 0 1 0
26 0 0 0 0 1 0
27 0 0 0 0 1 0
28 0 0 0 0 0 1
29 0 0 0 1 0 0
30 0 0 0 0 1 0
31 0 0 1 0 0 0
32 0 0 1 0 0 0
33 1 0 0 0 0 0
34 1 0 0 0 0 0
35 1 0 0 0 0 0
36 0 0 0 0 1 0
37 0 0 1 0 0 0
38 0 0 0 0 0 0
39 0 0 0 0 0 1
40 0 0 0 0 1 0
41 0 1 0 0 0 0
42 0 0 0 0 0 0
43 0 0 0 0 0 0
44 0 0 0 0 0 0
45 0 0 0 0 0 0
NON_CF.CTRL batch1 batch2 batch3 batch4 batch5 batch6 age sexM
1 1 0 0 1 0 0 0 -0.25908722 1
2 0 0 0 1 0 0 0 -0.09390014 1
3 0 0 0 1 0 0 0 -0.11514787 0
4 0 0 0 0 1 0 0 -0.04414710 0
5 0 0 0 0 1 0 0 0.14288337 1
6 0 0 0 0 0 1 0 -0.07296080 0
7 1 0 0 1 0 0 0 0.14645883 1
8 0 0 0 0 0 1 0 0.55970971 1
9 0 0 0 1 0 0 0 1.57438357 0
10 0 0 0 1 0 0 0 1.59938302 1
11 0 0 0 0 0 1 0 1.97206312 0
12 0 0 0 0 0 1 0 2.38835941 1
13 0 1 0 0 0 0 0 2.29572302 0
14 0 1 0 0 0 0 0 2.33608770 1
15 0 1 0 0 0 0 0 2.29801547 1
16 0 0 0 0 0 1 0 2.57902140 0
17 0 1 0 0 0 0 0 2.58232503 0
18 1 0 0 0 0 1 0 0.13210354 1
19 0 1 0 0 0 0 0 2.58890969 0
20 0 1 0 0 0 0 0 2.55836829 0
21 0 1 0 0 0 0 0 2.56706530 0
22 0 1 0 0 0 0 0 2.57305573 1
23 0 0 0 0 0 0 1 -0.93432383 0
24 0 0 0 0 0 0 1 0.09187369 0
25 0 0 0 0 0 0 1 1.04091644 0
26 0 0 0 0 0 0 1 0.08070438 1
27 0 0 0 0 0 0 1 0.99405890 1
28 0 0 0 0 0 0 1 -0.05642543 0
29 0 0 0 0 0 0 1 1.17649766 0
30 0 0 1 0 0 0 0 1.55970971 0
31 0 0 1 0 0 0 0 2.19301559 0
32 0 0 1 0 0 0 0 2.29801547 0
33 0 0 1 0 0 0 0 1.57039639 1
34 0 0 1 0 0 0 0 2.02060327 1
35 0 0 1 0 0 0 0 2.34855840 1
36 0 0 0 0 1 0 0 1.97307024 0
37 0 0 0 0 1 0 0 2.62971590 0
38 1 0 0 0 0 0 1 0.29237837 1
39 0 0 0 0 0 0 0 1.58014548 1
40 0 0 0 0 0 0 0 1.58014548 1
41 0 0 0 0 0 0 0 1.59931779 1
42 1 0 0 0 0 0 0 1.58496250 1
43 1 0 1 0 0 0 0 3.06991870 0
44 1 0 0 1 0 0 0 2.42046210 1
45 1 0 0 1 0 0 0 2.23560118 0
attr(,"assign")
[1] 1 1 1 1 1 1 1 2 2 2 2 2 2 3 4
attr(,"contrasts")
attr(,"contrasts")$group
[1] "contr.treatment"
attr(,"contrasts")$batch
[1] "contr.treatment"
attr(,"contrasts")$sex
[1] "contr.treatment"Create the contrast matrix.
contr <- makeContrasts(CF.NO_MODvNON_CF.CTRL = 0.5*(CF.NO_MOD.M + CF.NO_MOD.S) - NON_CF.CTRL,
CF.IVAvCF.NO_MOD = 0.5*(CF.IVA.S + CF.IVA.M) - 0.5*(CF.NO_MOD.S + CF.NO_MOD.M),
CF.LUMA_IVAvCF.NO_MOD = 0.5*(CF.LUMA_IVA.S + CF.LUMA_IVA.M) - 0.5*(CF.NO_MOD.S + CF.NO_MOD.M),
CF.NO_MOD.SvCF.NO_MOD.M = CF.NO_MOD.S - CF.NO_MOD.M,
levels = design)
contr Contrasts
Levels CF.NO_MODvNON_CF.CTRL CF.IVAvCF.NO_MOD CF.LUMA_IVAvCF.NO_MOD
CF.IVA.M 0.0 0.5 0.0
CF.IVA.S 0.0 0.5 0.0
CF.LUMA_IVA.M 0.0 0.0 0.5
CF.LUMA_IVA.S 0.0 0.0 0.5
CF.NO_MOD.M 0.5 -0.5 -0.5
CF.NO_MOD.S 0.5 -0.5 -0.5
NON_CF.CTRL -1.0 0.0 0.0
batch1 0.0 0.0 0.0
batch2 0.0 0.0 0.0
batch3 0.0 0.0 0.0
batch4 0.0 0.0 0.0
batch5 0.0 0.0 0.0
batch6 0.0 0.0 0.0
age 0.0 0.0 0.0
sexM 0.0 0.0 0.0
Contrasts
Levels CF.NO_MOD.SvCF.NO_MOD.M
CF.IVA.M 0
CF.IVA.S 0
CF.LUMA_IVA.M 0
CF.LUMA_IVA.S 0
CF.NO_MOD.M -1
CF.NO_MOD.S 1
NON_CF.CTRL 0
batch1 0
batch2 0
batch3 0
batch4 0
batch5 0
batch6 0
age 0
sexM 0Add random effect for samples from the same individual.
dupcor <- duplicateCorrelation(props$TransformedProps, design=design,
block=participant)
dupcor$consensus.correlation
[1] 0.3277853
$cor
[1] 0.3277853
$atanh.correlations
[1] 0.7768632 0.9562918 0.3072309 -0.4701327 1.4513133 0.4547148
[7] 0.3994249 1.0196590 1.1708055 -0.5159576 -0.5360603 0.8789047
[13] 1.2095895 -0.1972817 -0.5360603 -0.1313298 1.4752100 -0.5360603
[19] 0.6293269 0.7180931 0.9682816 0.4294997 0.0973840 -0.3852555
[25] -0.3808671 -0.5360603 0.9117043Fit the model.
fit <- lmFit(props$TransformedProps, design=design, block=participant,
correlation=dupcor$consensus)
fit2 <- contrasts.fit(fit, contr)
fit2 <- eBayes(fit2, robust=TRUE, trend=FALSE)
pvalue <- 0.05
summary(decideTests(fit2, p.value = pvalue)) CF.NO_MODvNON_CF.CTRL CF.IVAvCF.NO_MOD CF.LUMA_IVAvCF.NO_MOD
Down 0 0 0
NotSig 27 27 27
Up 0 0 0
CF.NO_MOD.SvCF.NO_MOD.M
Down 0
NotSig 27
Up 0Results
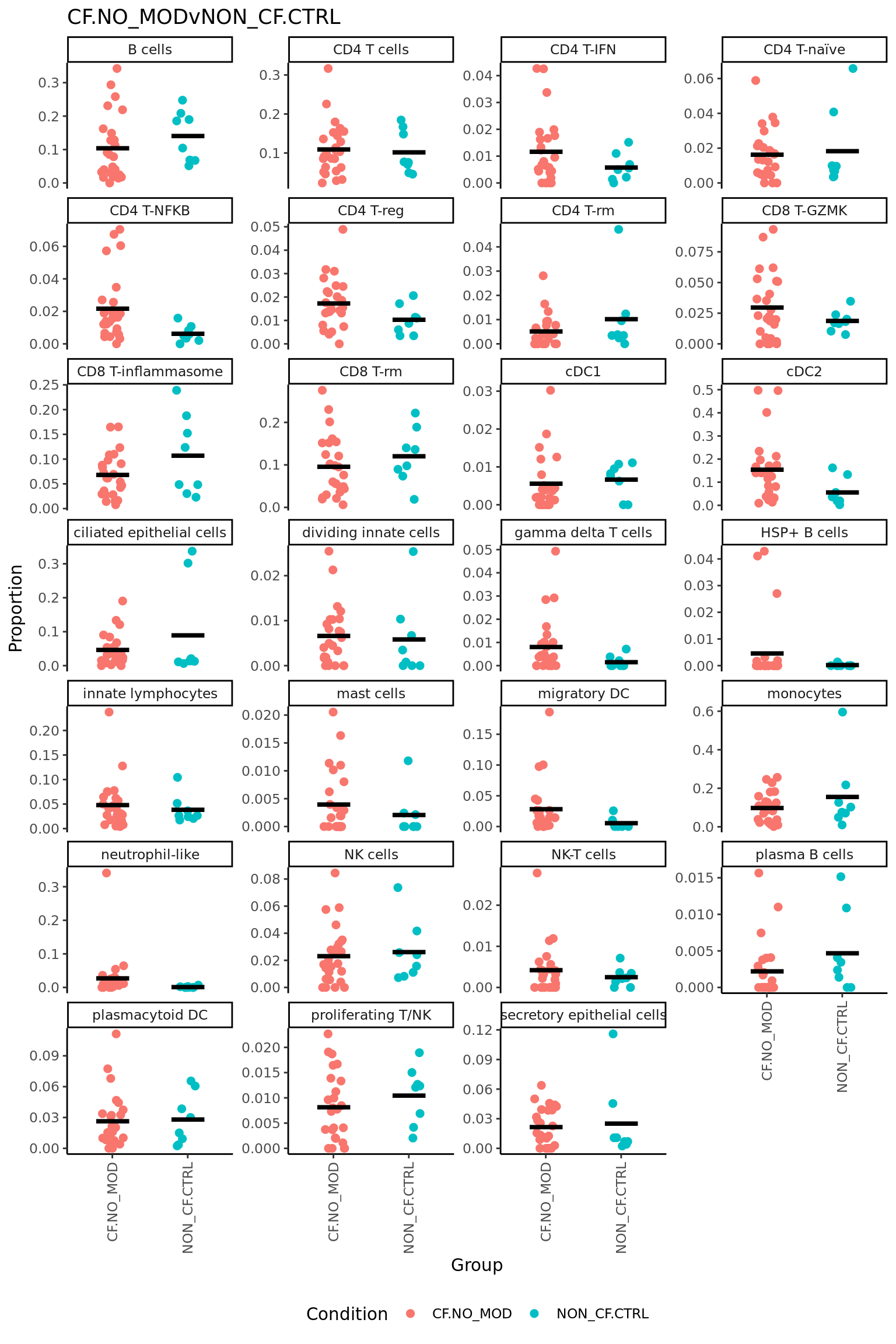
p <- vector("list", ncol(contr))
for(i in 1:ncol(contr)){
print(knitr::kable(topTable(fit2, coef = i, number = Inf),
caption = colnames(contr)[i]))
props$Proportions %>% data.frame %>%
left_join(info,
by = c("sample" = "sample.id")) %>%
mutate(Group = Group_severity) %>%
dplyr::filter(Group %in% names(contr[,i])[abs(contr[, i]) > 0]) -> dat
if(length(unique(dat$Group)) > 2) dat$Group <- str_remove(dat$Group, ".(M|S)$")
ggplot(dat, aes(x = Group,
y = Freq,
colour = Group,
group = Group)) +
geom_jitter(stat = "identity",
width = 0.15,
size = 2) +
stat_summary(geom = "point",
fun.y = "mean",
col = "black",
shape = "_",
size = 14) +
theme_classic() +
theme(axis.text.x = element_text(angle = 90,
hjust = 1,
vjust = 0.5),
legend.position = "bottom",
legend.direction = "horizontal") +
labs(x = "Group", y = "Proportion",
colour = "Condition") +
facet_wrap(~clusters, scales = "free_y", ncol = 4) +
ggtitle(colnames(contr)[i]) -> p[[i]]
print(p[[i]])
}| logFC | AveExpr | t | P.Value | adj.P.Val | B | |
|---|---|---|---|---|---|---|
| CD4 T-NFKB | 0.0847007 | 0.1287793 | 2.8538852 | 0.0072978 | 0.1282161 | -2.429406 |
| monocytes | -0.1632164 | 0.3123190 | -2.7509825 | 0.0094975 | 0.1282161 | -2.650007 |
| NK-T cells | 0.0486813 | 0.0408610 | 2.5379077 | 0.0158943 | 0.1303471 | -3.081849 |
| CD4 T-reg | 0.0554120 | 0.1286141 | 2.4558708 | 0.0193107 | 0.1303471 | -3.243228 |
| gamma delta T cells | 0.0494510 | 0.0577517 | 1.7135168 | 0.0957074 | 0.4464685 | -4.523912 |
| plasmacytoid DC | 0.0578468 | 0.1424289 | 1.5941695 | 0.1201376 | 0.4464685 | -4.695605 |
| innate lymphocytes | 0.0683991 | 0.1995245 | 1.5785830 | 0.1236760 | 0.4464685 | -4.717255 |
| CD4 T cells | 0.0545011 | 0.3376805 | 1.5421157 | 0.1322870 | 0.4464685 | -4.767199 |
| CD8 T-GZMK | 0.0406035 | 0.1365863 | 1.2167195 | 0.2320758 | 0.5659647 | -5.167206 |
| CD4 T-IFN | 0.0339050 | 0.0956091 | 1.1903718 | 0.2421316 | 0.5659647 | -5.195879 |
| secretory epithelial cells | 0.0413694 | 0.1310189 | 1.1848542 | 0.2442775 | 0.5659647 | -5.201811 |
| neutrophil-like | 0.0503802 | 0.0939267 | 1.1664396 | 0.2515399 | 0.5659647 | -5.221426 |
| proliferating T/NK | 0.0250960 | 0.0884279 | 1.0865341 | 0.2848732 | 0.5701673 | -5.303261 |
| CD8 T-rm | -0.0517457 | 0.3172748 | -1.0282531 | 0.3111704 | 0.5701673 | -5.359474 |
| HSP+ B cells | 0.0264486 | 0.0190050 | 1.0160713 | 0.3167596 | 0.5701673 | -5.370950 |
| plasma B cells | -0.0194721 | 0.0328307 | -0.8142958 | 0.4211329 | 0.7106617 | -5.541041 |
| cDC1 | 0.0141421 | 0.0623532 | 0.5700950 | 0.5723552 | 0.8533862 | -5.698464 |
| CD8 T-inflammasome | -0.0240626 | 0.2723283 | -0.5456907 | 0.5888352 | 0.8533862 | -5.711217 |
| NK cells | 0.0170900 | 0.1351729 | 0.5006215 | 0.6198575 | 0.8533862 | -5.733327 |
| CD4 T-naïve | 0.0130592 | 0.1115006 | 0.4518533 | 0.6542393 | 0.8533862 | -5.755139 |
| ciliated epithelial cells | 0.0259043 | 0.2001168 | 0.3819383 | 0.7049101 | 0.8533862 | -5.782535 |
| migratory DC | -0.0115279 | 0.1021530 | -0.3601260 | 0.7209767 | 0.8533862 | -5.790180 |
| cDC2 | -0.0190291 | 0.3135402 | -0.3521023 | 0.7269586 | 0.8533862 | -5.792852 |
| B cells | -0.0220155 | 0.3234933 | -0.3089427 | 0.7592711 | 0.8541800 | -5.806303 |
| dividing innate cells | -0.0065947 | 0.0608827 | -0.2649911 | 0.7926136 | 0.8560227 | -5.818218 |
| CD4 T-rm | 0.0026089 | 0.0701179 | 0.1505950 | 0.8811836 | 0.9150753 | -5.840670 |
| mast cells | -0.0003023 | 0.0401931 | -0.0138387 | 0.9890394 | 0.9890394 | -5.851302 |
| logFC | AveExpr | t | P.Value | adj.P.Val | B | |
|---|---|---|---|---|---|---|
| NK-T cells | -0.0572207 | 0.0408610 | -2.5330118 | 0.0160815 | 0.4342005 | -3.034135 |
| CD8 T-GZMK | -0.0810316 | 0.1365863 | -2.0618275 | 0.0469210 | 0.6334341 | -3.878364 |
| CD4 T-naïve | -0.0451394 | 0.1115006 | -1.3261927 | 0.1936014 | 0.9406935 | -4.919067 |
| plasmacytoid DC | -0.0555689 | 0.1424289 | -1.3003433 | 0.2022144 | 0.9406935 | -4.948539 |
| CD4 T-IFN | 0.0334608 | 0.0956091 | 0.9975295 | 0.3255425 | 0.9406935 | -5.254221 |
| CD8 T-rm | 0.0546817 | 0.3172748 | 0.9226540 | 0.3627605 | 0.9406935 | -5.318150 |
| CD8 T-inflammasome | 0.0436714 | 0.2723283 | 0.8409526 | 0.4062402 | 0.9406935 | -5.382661 |
| CD4 T cells | -0.0337599 | 0.3376805 | -0.8111161 | 0.4229314 | 0.9406935 | -5.404772 |
| cDC1 | -0.0231483 | 0.0623532 | -0.7923597 | 0.4336358 | 0.9406935 | -5.418281 |
| CD4 T-rm | -0.0136891 | 0.0701179 | -0.6709533 | 0.5067755 | 0.9406935 | -5.498342 |
| gamma delta T cells | -0.0227682 | 0.0577517 | -0.6699062 | 0.5074345 | 0.9406935 | -5.498977 |
| HSP+ B cells | -0.0193460 | 0.0190050 | -0.6310791 | 0.5322015 | 0.9406935 | -5.521826 |
| plasma B cells | -0.0148971 | 0.0328307 | -0.5289833 | 0.6002480 | 0.9406935 | -5.575545 |
| B cells | -0.0427921 | 0.3234933 | -0.5098993 | 0.6134525 | 0.9406935 | -5.584520 |
| CD4 T-NFKB | -0.0168516 | 0.1287793 | -0.4821267 | 0.6328000 | 0.9406935 | -5.597091 |
| ciliated epithelial cells | -0.0347923 | 0.2001168 | -0.4355870 | 0.6659197 | 0.9406935 | -5.616515 |
| CD4 T-reg | -0.0110401 | 0.1286141 | -0.4154744 | 0.6804008 | 0.9406935 | -5.624346 |
| secretory epithelial cells | 0.0137575 | 0.1310189 | 0.3345764 | 0.7399964 | 0.9406935 | -5.652043 |
| mast cells | 0.0074871 | 0.0401931 | 0.2910805 | 0.7727551 | 0.9406935 | -5.664483 |
| migratory DC | 0.0106213 | 0.1021530 | 0.2817408 | 0.7798472 | 0.9406935 | -5.666930 |
| neutrophil-like | 0.0137353 | 0.0939267 | 0.2700294 | 0.7887672 | 0.9406935 | -5.669886 |
| NK cells | -0.0093315 | 0.1351729 | -0.2321090 | 0.8178424 | 0.9406935 | -5.678600 |
| cDC2 | 0.0134292 | 0.3135402 | 0.2109942 | 0.8341652 | 0.9406935 | -5.682876 |
| dividing innate cells | 0.0060124 | 0.0608827 | 0.2051439 | 0.8386809 | 0.9406935 | -5.683998 |
| proliferating T/NK | 0.0044502 | 0.0884279 | 0.1636007 | 0.8710125 | 0.9406935 | -5.691015 |
| innate lymphocytes | -0.0049377 | 0.1995245 | -0.0967643 | 0.9234808 | 0.9589993 | -5.698991 |
| monocytes | 0.0027904 | 0.3123190 | 0.0399352 | 0.9683809 | 0.9683809 | -5.702552 |
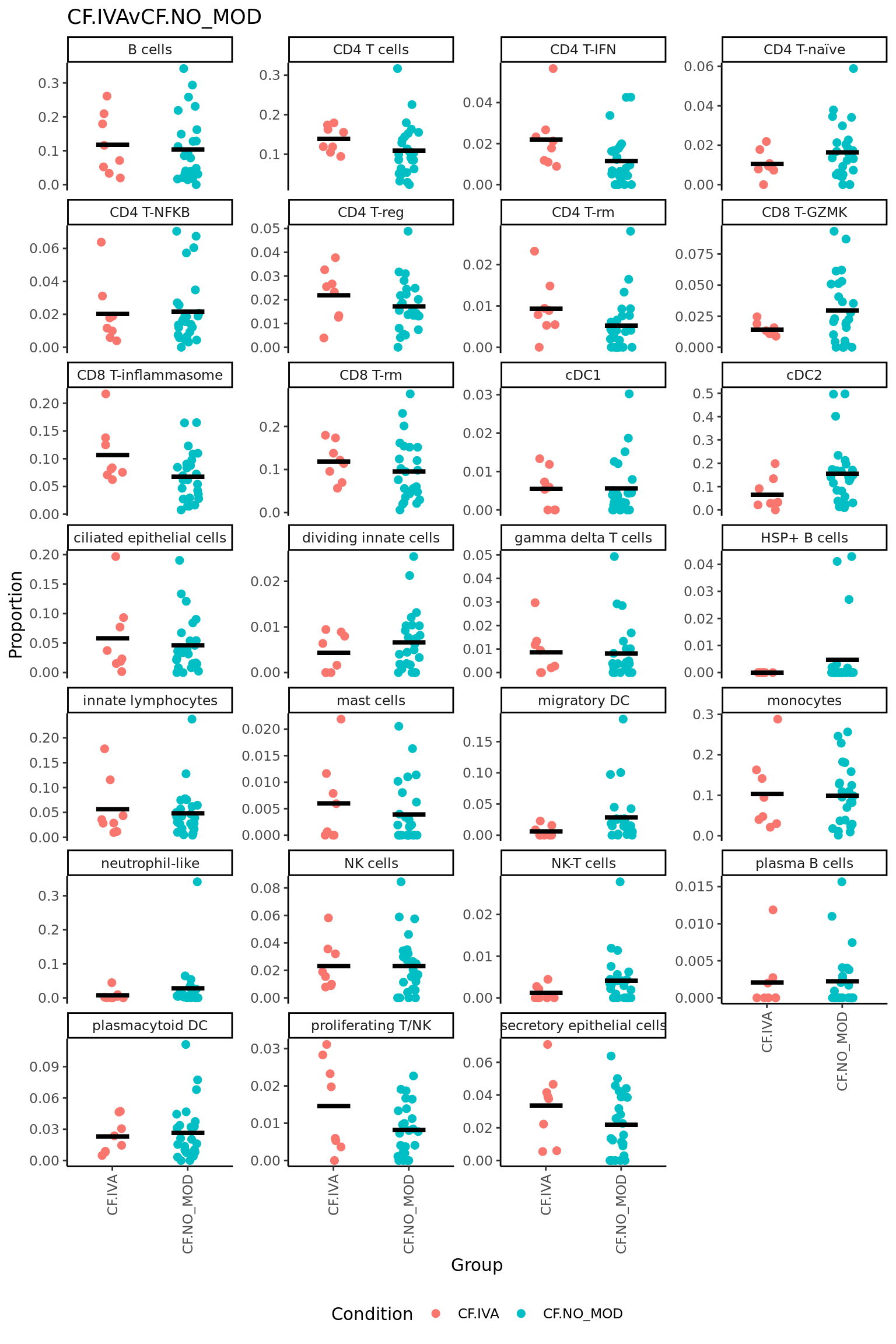
| logFC | AveExpr | t | P.Value | adj.P.Val | B | |
|---|---|---|---|---|---|---|
| neutrophil-like | -0.1295546 | 0.0939267 | -2.4343057 | 0.0203138 | 0.4226372 | -3.207923 |
| HSP+ B cells | -0.0720366 | 0.0190050 | -2.2459166 | 0.0313065 | 0.4226372 | -3.546189 |
| mast cells | -0.0427362 | 0.0401931 | -1.5879729 | 0.1215343 | 0.6931957 | -4.563749 |
| innate lymphocytes | 0.0832526 | 0.1995245 | 1.5593161 | 0.1281668 | 0.6931957 | -4.601586 |
| CD4 T cells | 0.0639478 | 0.3376805 | 1.4684396 | 0.1511611 | 0.6931957 | -4.717671 |
| gamma delta T cells | -0.0518416 | 0.0577517 | -1.4578451 | 0.1540435 | 0.6931957 | -4.730813 |
| CD4 T-IFN | 0.0459208 | 0.0956091 | 1.3084190 | 0.1994928 | 0.7694721 | -4.907252 |
| CD8 T-rm | 0.0640722 | 0.3172748 | 1.0332722 | 0.3088506 | 0.9060566 | -5.186823 |
| B cells | 0.0799948 | 0.3234933 | 0.9110263 | 0.3687660 | 0.9060566 | -5.291434 |
| CD4 T-naïve | 0.0319332 | 0.1115006 | 0.8966884 | 0.3761800 | 0.9060566 | -5.302962 |
| cDC1 | 0.0255733 | 0.0623532 | 0.8366399 | 0.4086272 | 0.9060566 | -5.349064 |
| plasmacytoid DC | -0.0347074 | 0.1424289 | -0.7762404 | 0.4429647 | 0.9060566 | -5.392353 |
| CD4 T-reg | 0.0191841 | 0.1286141 | 0.6900214 | 0.4948562 | 0.9060566 | -5.448734 |
| migratory DC | 0.0269655 | 0.1021530 | 0.6836450 | 0.4988245 | 0.9060566 | -5.452649 |
| NK cells | 0.0284515 | 0.1351729 | 0.6763840 | 0.5033648 | 0.9060566 | -5.457064 |
| plasma B cells | -0.0120072 | 0.0328307 | -0.4075032 | 0.6861887 | 0.9971132 | -5.588003 |
| CD4 T-NFKB | -0.0123239 | 0.1287793 | -0.3369898 | 0.7381925 | 0.9971132 | -5.611722 |
| ciliated epithelial cells | -0.0250460 | 0.2001168 | -0.2996953 | 0.7662542 | 0.9971132 | -5.622450 |
| proliferating T/NK | -0.0075540 | 0.0884279 | -0.2654214 | 0.7922849 | 0.9971132 | -5.631232 |
| monocytes | 0.0144396 | 0.3123190 | 0.1975138 | 0.8446146 | 0.9971132 | -5.645469 |
| dividing innate cells | -0.0045144 | 0.0608827 | -0.1472177 | 0.8838282 | 0.9971132 | -5.653337 |
| secretory epithelial cells | -0.0059562 | 0.1310189 | -0.1384436 | 0.8907052 | 0.9971132 | -5.654474 |
| NK-T cells | -0.0032617 | 0.0408610 | -0.1379986 | 0.8910542 | 0.9971132 | -5.654530 |
| CD8 T-inflammasome | 0.0070011 | 0.2723283 | 0.1288516 | 0.8982330 | 0.9971132 | -5.655637 |
| CD4 T-rm | -0.0012765 | 0.0701179 | -0.0597979 | 0.9526661 | 0.9971132 | -5.661548 |
| cDC2 | -0.0007636 | 0.3135402 | -0.0114669 | 0.9909187 | 0.9971132 | -5.663111 |
| CD8 T-GZMK | 0.0001499 | 0.1365863 | 0.0036448 | 0.9971132 | 0.9971132 | -5.663165 |
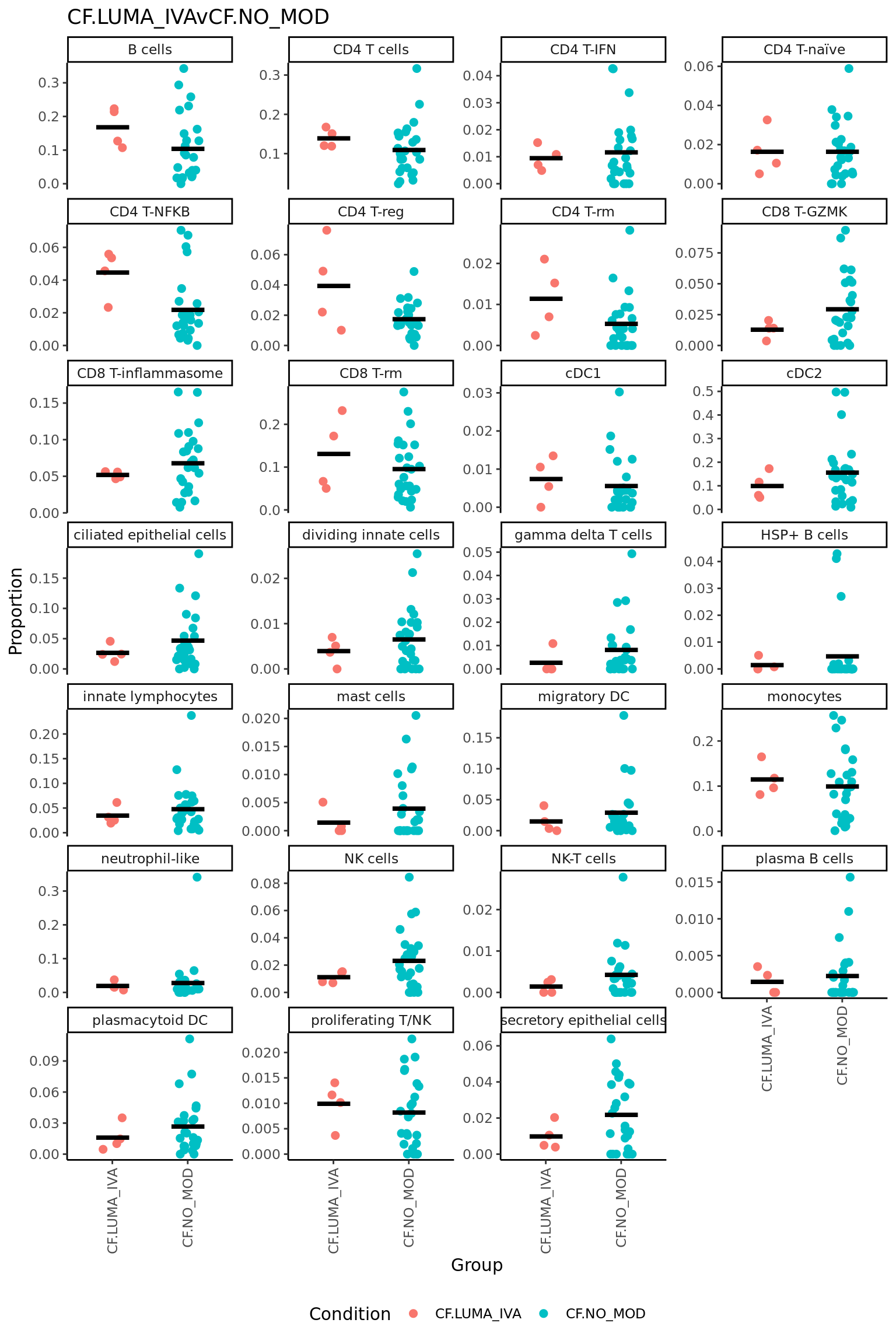
| logFC | AveExpr | t | P.Value | adj.P.Val | B | |
|---|---|---|---|---|---|---|
| CD4 T-IFN | -0.0667822 | 0.0956091 | -2.5517560 | 0.0153756 | 0.1764439 | -3.093739 |
| CD8 T-rm | -0.1154065 | 0.3172748 | -2.4958361 | 0.0176380 | 0.1764439 | -3.208159 |
| cDC2 | -0.1215812 | 0.3135402 | -2.4483675 | 0.0197258 | 0.1764439 | -3.302140 |
| B cells | 0.1523514 | 0.3234933 | 2.3267852 | 0.0261398 | 0.1764439 | -3.537235 |
| HSP+ B cells | 0.0518879 | 0.0190050 | 2.1694357 | 0.0371204 | 0.2004500 | -3.827852 |
| monocytes | -0.1056514 | 0.3123190 | -1.9380249 | 0.0610535 | 0.2300316 | -4.229961 |
| neutrophil-like | 0.0752961 | 0.0939267 | 1.8972970 | 0.0662938 | 0.2300316 | -4.296433 |
| ciliated epithelial cells | 0.1174431 | 0.2001168 | 1.8845555 | 0.0681575 | 0.2300316 | -4.317643 |
| migratory DC | -0.0495892 | 0.1021530 | -1.6859701 | 0.1009434 | 0.2848108 | -4.625669 |
| mast cells | 0.0333741 | 0.0401931 | 1.6630210 | 0.1054855 | 0.2848108 | -4.659491 |
| CD8 T-inflammasome | -0.0572727 | 0.2723283 | -1.4135512 | 0.1665694 | 0.4088520 | -5.001459 |
| NK cells | 0.0394335 | 0.1351729 | 1.2571682 | 0.2172459 | 0.4784539 | -5.190846 |
| plasma B cells | 0.0268340 | 0.0328307 | 1.2212811 | 0.2303667 | 0.4784539 | -5.231485 |
| cDC1 | 0.0250117 | 0.0623532 | 1.0973292 | 0.2801956 | 0.5403772 | -5.363548 |
| innate lymphocytes | -0.0415586 | 0.1995245 | -1.0438490 | 0.3039082 | 0.5470347 | -5.416489 |
| plasmacytoid DC | 0.0275118 | 0.1424289 | 0.8251518 | 0.4150278 | 0.6424480 | -5.606979 |
| dividing innate cells | 0.0186079 | 0.0608827 | 0.8137553 | 0.4214382 | 0.6424480 | -5.615741 |
| CD4 T cells | 0.0254022 | 0.3376805 | 0.7822433 | 0.4394766 | 0.6424480 | -5.639359 |
| gamma delta T cells | 0.0201392 | 0.0577517 | 0.7594804 | 0.4527903 | 0.6424480 | -5.655861 |
| CD4 T-naïve | 0.0189718 | 0.1115006 | 0.7144105 | 0.4798416 | 0.6424480 | -5.687146 |
| NK-T cells | 0.0120250 | 0.0408610 | 0.6822712 | 0.4996818 | 0.6424480 | -5.708323 |
| CD4 T-reg | 0.0129865 | 0.1286141 | 0.6264053 | 0.5352254 | 0.6568676 | -5.742878 |
| CD8 T-GZMK | 0.0170957 | 0.1365863 | 0.5575382 | 0.5808061 | 0.6818159 | -5.781507 |
| proliferating T/NK | 0.0108688 | 0.0884279 | 0.5121304 | 0.6118650 | 0.6883481 | -5.804565 |
| CD4 T-NFKB | 0.0114954 | 0.1287793 | 0.4215340 | 0.6760139 | 0.7300951 | -5.844805 |
| secretory epithelial cells | 0.0074881 | 0.1310189 | 0.2334095 | 0.8168407 | 0.8482576 | -5.903609 |
| CD4 T-rm | 0.0020995 | 0.0701179 | 0.1318942 | 0.8958441 | 0.8958441 | -5.921353 |
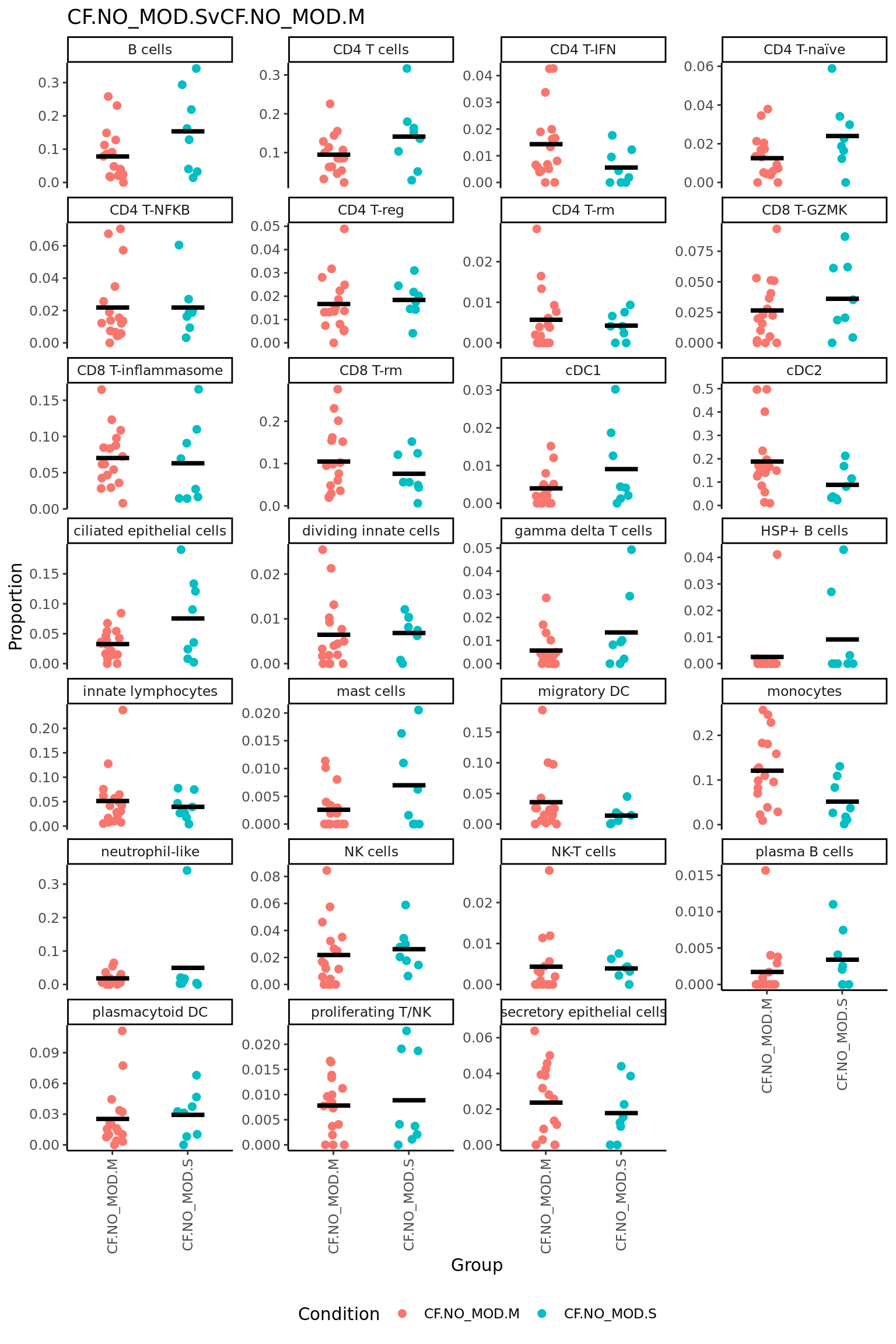
Session info
sessioninfo::session_info()─ Session info ───────────────────────────────────────────────────────────────
setting value
version R version 4.3.3 (2024-02-29)
os Ubuntu 22.04.4 LTS
system x86_64, linux-gnu
ui X11
language (EN)
collate en_AU.UTF-8
ctype en_AU.UTF-8
tz Etc/UTC
date 2024-12-31
pandoc 3.1.1 @ /usr/lib/rstudio-server/bin/quarto/bin/tools/ (via rmarkdown)
─ Packages ───────────────────────────────────────────────────────────────────
! package * version date (UTC) lib source
P abind 1.4-5 2016-07-21 [?] RSPM (R 4.3.0)
P AnnotationDbi * 1.64.1 2023-11-03 [?] Bioconductor
P backports 1.4.1 2021-12-13 [?] RSPM (R 4.3.0)
P base64enc 0.1-3 2015-07-28 [?] RSPM (R 4.3.0)
P Biobase * 2.62.0 2023-10-24 [?] Bioconductor
P BiocGenerics * 0.48.1 2023-11-01 [?] Bioconductor
P BiocManager 1.30.22 2023-08-08 [?] RSPM (R 4.3.0)
P Biostrings 2.70.2 2024-01-28 [?] Bioconductor 3.18 (R 4.3.3)
P bit 4.0.5 2022-11-15 [?] RSPM (R 4.3.0)
P bit64 4.0.5 2020-08-30 [?] RSPM (R 4.3.0)
P bitops 1.0-7 2021-04-24 [?] RSPM (R 4.3.0)
P blob 1.2.4 2023-03-17 [?] RSPM (R 4.3.0)
P bslib 0.6.1 2023-11-28 [?] RSPM (R 4.3.0)
P cachem 1.0.8 2023-05-01 [?] RSPM (R 4.3.0)
P callr 3.7.3 2022-11-02 [?] RSPM (R 4.3.0)
P checkmate 2.3.1 2023-12-04 [?] RSPM (R 4.3.0)
P circlize 0.4.15 2022-05-10 [?] RSPM (R 4.3.0)
P cli 3.6.2 2023-12-11 [?] RSPM (R 4.3.0)
P clue 0.3-65 2023-09-23 [?] RSPM (R 4.3.0)
P cluster 2.1.6 2023-12-01 [?] CRAN (R 4.3.2)
P clustree * 0.5.1 2023-11-05 [?] RSPM (R 4.3.0)
P codetools 0.2-19 2023-02-01 [?] CRAN (R 4.2.2)
P colorspace 2.1-0 2023-01-23 [?] RSPM (R 4.3.0)
P ComplexHeatmap 2.18.0 2023-10-24 [?] Bioconductor
P cowplot 1.1.3 2024-01-22 [?] RSPM (R 4.3.0)
P crayon 1.5.2 2022-09-29 [?] RSPM (R 4.3.0)
P data.table 1.15.0 2024-01-30 [?] RSPM (R 4.3.0)
P DBI 1.2.1 2024-01-12 [?] RSPM (R 4.3.0)
P DelayedArray 0.28.0 2023-10-24 [?] Bioconductor
P deldir 2.0-2 2023-11-23 [?] RSPM (R 4.3.0)
P dendextend 1.17.1 2023-03-25 [?] RSPM (R 4.3.0)
P digest 0.6.34 2024-01-11 [?] RSPM (R 4.3.0)
P dittoSeq * 1.14.2 2024-02-09 [?] Bioconductor 3.18 (R 4.3.3)
P doParallel 1.0.17 2022-02-07 [?] RSPM (R 4.3.0)
P dplyr * 1.1.4 2023-11-17 [?] RSPM (R 4.3.0)
P dynamicTreeCut 1.63-1 2016-03-11 [?] RSPM (R 4.3.0)
P edgeR * 4.0.15 2024-02-11 [?] Bioconductor 3.18 (R 4.3.3)
P ellipsis 0.3.2 2021-04-29 [?] RSPM (R 4.3.0)
P evaluate 0.23 2023-11-01 [?] RSPM (R 4.3.0)
P fansi 1.0.6 2023-12-08 [?] RSPM (R 4.3.0)
P farver 2.1.1 2022-07-06 [?] RSPM (R 4.3.0)
P fastcluster 1.2.6 2024-01-12 [?] RSPM (R 4.3.0)
P fastmap 1.1.1 2023-02-24 [?] RSPM (R 4.3.0)
P fitdistrplus 1.1-11 2023-04-25 [?] RSPM (R 4.3.0)
P forcats * 1.0.0 2023-01-29 [?] RSPM (R 4.3.0)
P foreach 1.5.2 2022-02-02 [?] RSPM (R 4.3.0)
P foreign 0.8-86 2023-11-28 [?] CRAN (R 4.3.2)
P Formula 1.2-5 2023-02-24 [?] RSPM (R 4.3.0)
P fs 1.6.3 2023-07-20 [?] RSPM (R 4.3.0)
P future 1.33.1 2023-12-22 [?] RSPM (R 4.3.0)
P future.apply 1.11.1 2023-12-21 [?] RSPM (R 4.3.0)
P generics 0.1.3 2022-07-05 [?] RSPM (R 4.3.0)
P GenomeInfoDb * 1.38.6 2024-02-08 [?] Bioconductor 3.18 (R 4.3.3)
P GenomeInfoDbData 1.2.11 2024-04-23 [?] Bioconductor
P GenomicRanges * 1.54.1 2023-10-29 [?] Bioconductor
P GetoptLong 1.0.5 2020-12-15 [?] RSPM (R 4.3.0)
P getPass 0.2-4 2023-12-10 [?] RSPM (R 4.3.0)
P ggforce 0.4.2 2024-02-19 [?] RSPM (R 4.3.0)
P ggplot2 * 3.5.0 2024-02-23 [?] RSPM (R 4.3.0)
P ggraph * 2.2.0 2024-02-27 [?] RSPM (R 4.3.0)
P ggrepel 0.9.5 2024-01-10 [?] RSPM (R 4.3.0)
P ggridges 0.5.6 2024-01-23 [?] RSPM (R 4.3.0)
P git2r 0.33.0 2023-11-26 [?] RSPM (R 4.3.0)
P glmGamPoi * 1.14.3 2024-02-11 [?] Bioconductor 3.18 (R 4.3.3)
P GlobalOptions 0.1.2 2020-06-10 [?] RSPM (R 4.3.0)
P globals 0.16.2 2022-11-21 [?] RSPM (R 4.3.0)
P glue * 1.7.0 2024-01-09 [?] RSPM (R 4.3.0)
P GO.db 3.18.0 2024-04-23 [?] Bioconductor
P goftest 1.2-3 2021-10-07 [?] RSPM (R 4.3.0)
P graphlayouts 1.1.0 2024-01-19 [?] RSPM (R 4.3.0)
P gridExtra 2.3 2017-09-09 [?] RSPM (R 4.3.0)
P gtable 0.3.4 2023-08-21 [?] RSPM (R 4.3.0)
P here * 1.0.1 2020-12-13 [?] RSPM (R 4.3.0)
P highr 0.10 2022-12-22 [?] RSPM (R 4.3.0)
P Hmisc 5.1-1 2023-09-12 [?] RSPM (R 4.3.0)
P hms 1.1.3 2023-03-21 [?] RSPM (R 4.3.0)
P htmlTable 2.4.2 2023-10-29 [?] RSPM (R 4.3.0)
P htmltools 0.5.7 2023-11-03 [?] RSPM (R 4.3.0)
P htmlwidgets 1.6.4 2023-12-06 [?] RSPM (R 4.3.0)
P httpuv 1.6.14 2024-01-26 [?] RSPM (R 4.3.0)
P httr 1.4.7 2023-08-15 [?] RSPM (R 4.3.0)
P ica 1.0-3 2022-07-08 [?] RSPM (R 4.3.0)
P igraph 2.0.1.1 2024-01-30 [?] RSPM (R 4.3.0)
P impute 1.76.0 2023-10-24 [?] Bioconductor
P IRanges * 2.36.0 2023-10-24 [?] Bioconductor
P irlba 2.3.5.1 2022-10-03 [?] RSPM (R 4.3.0)
P iterators 1.0.14 2022-02-05 [?] RSPM (R 4.3.0)
P jquerylib 0.1.4 2021-04-26 [?] RSPM (R 4.3.0)
P jsonlite 1.8.8 2023-12-04 [?] RSPM (R 4.3.0)
P KEGGREST 1.42.0 2023-10-24 [?] Bioconductor
P KernSmooth 2.23-24 2024-05-17 [?] RSPM (R 4.3.0)
P knitr 1.45 2023-10-30 [?] RSPM (R 4.3.0)
P labeling 0.4.3 2023-08-29 [?] RSPM (R 4.3.0)
P later 1.3.2 2023-12-06 [?] RSPM (R 4.3.0)
P lattice 0.22-5 2023-10-24 [?] CRAN (R 4.3.1)
P lazyeval 0.2.2 2019-03-15 [?] RSPM (R 4.3.0)
P leiden 0.4.3.1 2023-11-17 [?] RSPM (R 4.3.0)
P lifecycle 1.0.4 2023-11-07 [?] RSPM (R 4.3.0)
P limma * 3.58.1 2023-10-31 [?] Bioconductor
P listenv 0.9.1 2024-01-29 [?] RSPM (R 4.3.0)
P lmtest 0.9-40 2022-03-21 [?] RSPM (R 4.3.0)
P locfit 1.5-9.8 2023-06-11 [?] RSPM (R 4.3.0)
P lubridate * 1.9.3 2023-09-27 [?] RSPM (R 4.3.0)
P magrittr 2.0.3 2022-03-30 [?] RSPM (R 4.3.0)
P MASS 7.3-60.0.1 2024-01-13 [?] RSPM (R 4.3.0)
P Matrix 1.6-5 2024-01-11 [?] CRAN (R 4.3.3)
P MatrixGenerics * 1.14.0 2023-10-24 [?] Bioconductor
P matrixStats * 1.2.0 2023-12-11 [?] RSPM (R 4.3.0)
P memoise 2.0.1 2021-11-26 [?] RSPM (R 4.3.0)
P mime 0.12 2021-09-28 [?] RSPM (R 4.3.0)
P miniUI 0.1.1.1 2018-05-18 [?] RSPM (R 4.3.0)
P munsell 0.5.0 2018-06-12 [?] RSPM (R 4.3.0)
P nlme 3.1-164 2023-11-27 [?] RSPM (R 4.3.0)
P nnet 7.3-19 2023-05-03 [?] CRAN (R 4.3.1)
P org.Hs.eg.db * 3.18.0 2024-04-23 [?] Bioconductor
P paletteer * 1.6.0 2024-01-21 [?] RSPM (R 4.3.0)
P parallelly 1.37.0 2024-02-14 [?] RSPM (R 4.3.0)
P patchwork * 1.2.0 2024-01-08 [?] RSPM (R 4.3.0)
P pbapply 1.7-2 2023-06-27 [?] RSPM (R 4.3.0)
P pheatmap 1.0.12 2019-01-04 [?] RSPM (R 4.3.0)
P pillar 1.9.0 2023-03-22 [?] RSPM (R 4.3.0)
P pkgconfig 2.0.3 2019-09-22 [?] RSPM (R 4.3.0)
P plotly 4.10.4 2024-01-13 [?] RSPM (R 4.3.0)
P plyr 1.8.9 2023-10-02 [?] RSPM (R 4.3.0)
P png 0.1-8 2022-11-29 [?] RSPM (R 4.3.0)
P polyclip 1.10-6 2023-09-27 [?] RSPM (R 4.3.0)
P preprocessCore 1.64.0 2023-10-24 [?] Bioconductor
P processx 3.8.3 2023-12-10 [?] RSPM (R 4.3.0)
P progressr 0.14.0 2023-08-10 [?] RSPM (R 4.3.0)
P promises 1.2.1 2023-08-10 [?] RSPM (R 4.3.0)
P ps 1.7.6 2024-01-18 [?] RSPM (R 4.3.0)
P purrr * 1.0.2 2023-08-10 [?] RSPM (R 4.3.0)
P R6 2.5.1 2021-08-19 [?] RSPM (R 4.3.0)
P RANN 2.6.1 2019-01-08 [?] RSPM (R 4.3.0)
P RColorBrewer 1.1-3 2022-04-03 [?] RSPM (R 4.3.0)
P Rcpp 1.0.12 2024-01-09 [?] RSPM (R 4.3.0)
P RcppAnnoy 0.0.22 2024-01-23 [?] RSPM (R 4.3.0)
P RCurl 1.98-1.14 2024-01-09 [?] RSPM (R 4.3.0)
P readr * 2.1.5 2024-01-10 [?] RSPM (R 4.3.0)
P rematch2 2.1.2 2020-05-01 [?] RSPM (R 4.3.0)
renv 1.0.3 2023-09-19 [1] CRAN (R 4.3.3)
P reshape2 1.4.4 2020-04-09 [?] RSPM (R 4.3.0)
P reticulate 1.35.0 2024-01-31 [?] RSPM (R 4.3.0)
P rjson 0.2.21 2022-01-09 [?] RSPM (R 4.3.0)
P rlang 1.1.3 2024-01-10 [?] RSPM (R 4.3.0)
P rmarkdown 2.25 2023-09-18 [?] RSPM (R 4.3.0)
P ROCR 1.0-11 2020-05-02 [?] RSPM (R 4.3.0)
P rpart 4.1.23 2023-12-05 [?] RSPM (R 4.3.0)
P rprojroot 2.0.4 2023-11-05 [?] RSPM (R 4.3.0)
P RSQLite 2.3.5 2024-01-21 [?] RSPM (R 4.3.0)
P rstudioapi 0.15.0 2023-07-07 [?] RSPM (R 4.3.0)
P Rtsne 0.17 2023-12-07 [?] RSPM (R 4.3.0)
P S4Arrays 1.2.0 2023-10-24 [?] Bioconductor
P S4Vectors * 0.40.2 2023-11-23 [?] Bioconductor 3.18 (R 4.3.3)
P sass 0.4.8 2023-12-06 [?] RSPM (R 4.3.0)
P scales 1.3.0 2023-11-28 [?] RSPM (R 4.3.0)
P scattermore 1.2 2023-06-12 [?] RSPM (R 4.3.0)
sctransform 0.4.1 2023-10-19 [1] RSPM (R 4.3.0)
P sessioninfo 1.2.2 2021-12-06 [?] RSPM (R 4.3.0)
Seurat * 4.4.0 2024-04-25 [1] https://satijalab.r-universe.dev (R 4.3.3)
SeuratObject * 4.1.4 2024-04-25 [1] https://satijalab.r-universe.dev (R 4.3.3)
P shape 1.4.6 2021-05-19 [?] RSPM (R 4.3.0)
P shiny 1.8.0 2023-11-17 [?] RSPM (R 4.3.0)
P SingleCellExperiment * 1.24.0 2023-10-24 [?] Bioconductor
P sp 2.1-3 2024-01-30 [?] RSPM (R 4.3.0)
P SparseArray 1.2.4 2024-02-11 [?] Bioconductor 3.18 (R 4.3.3)
P spatstat.data 3.0-4 2024-01-15 [?] RSPM (R 4.3.0)
P spatstat.explore 3.2-6 2024-02-01 [?] RSPM (R 4.3.0)
P spatstat.geom 3.2-8 2024-01-26 [?] RSPM (R 4.3.0)
P spatstat.random 3.2-2 2023-11-29 [?] RSPM (R 4.3.0)
P spatstat.sparse 3.0-3 2023-10-24 [?] RSPM (R 4.3.0)
P spatstat.utils 3.0-4 2023-10-24 [?] RSPM (R 4.3.0)
P speckle * 1.2.0 2023-10-24 [?] Bioconductor
P statmod 1.5.0 2023-01-06 [?] RSPM (R 4.3.0)
P stringi 1.8.3 2023-12-11 [?] RSPM (R 4.3.0)
P stringr * 1.5.1 2023-11-14 [?] RSPM (R 4.3.0)
P SummarizedExperiment * 1.32.0 2023-10-24 [?] Bioconductor
P survival 3.7-0 2024-06-05 [?] RSPM (R 4.3.0)
P tensor 1.5 2012-05-05 [?] RSPM (R 4.3.0)
P tibble * 3.2.1 2023-03-20 [?] RSPM (R 4.3.0)
P tidygraph 1.3.1 2024-01-30 [?] RSPM (R 4.3.0)
P tidyHeatmap * 1.8.1 2022-05-20 [?] RSPM (R 4.3.3)
P tidyr * 1.3.1 2024-01-24 [?] RSPM (R 4.3.0)
P tidyselect 1.2.0 2022-10-10 [?] RSPM (R 4.3.0)
P tidyverse * 2.0.0 2023-02-22 [?] RSPM (R 4.3.0)
P timechange 0.3.0 2024-01-18 [?] RSPM (R 4.3.0)
P tweenr 2.0.3 2024-02-26 [?] RSPM (R 4.3.0)
P tzdb 0.4.0 2023-05-12 [?] RSPM (R 4.3.0)
P utf8 1.2.4 2023-10-22 [?] RSPM (R 4.3.0)
P uwot 0.1.16 2023-06-29 [?] RSPM (R 4.3.0)
P vctrs 0.6.5 2023-12-01 [?] RSPM (R 4.3.0)
P viridis 0.6.5 2024-01-29 [?] RSPM (R 4.3.0)
P viridisLite 0.4.2 2023-05-02 [?] RSPM (R 4.3.0)
P WGCNA 1.72-5 2023-12-07 [?] RSPM (R 4.3.3)
P whisker 0.4.1 2022-12-05 [?] RSPM (R 4.3.0)
P withr 3.0.0 2024-01-16 [?] RSPM (R 4.3.0)
P workflowr * 1.7.1 2023-08-23 [?] RSPM (R 4.3.0)
P xfun 0.42 2024-02-08 [?] RSPM (R 4.3.0)
P xtable 1.8-4 2019-04-21 [?] RSPM (R 4.3.0)
P XVector 0.42.0 2023-10-24 [?] Bioconductor
P yaml 2.3.8 2023-12-11 [?] RSPM (R 4.3.0)
P zlibbioc 1.48.0 2023-10-24 [?] Bioconductor
P zoo 1.8-12 2023-04-13 [?] RSPM (R 4.3.0)
[1] /mnt/allandata/jovana_data/paed-inflammation-CITEseq/renv/library/R-4.3/x86_64-pc-linux-gnu
[2] /home/jovana/.cache/R/renv/sandbox/R-4.3/x86_64-pc-linux-gnu/9a444a72
P ── Loaded and on-disk path mismatch.
──────────────────────────────────────────────────────────────────────────────
sessionInfo()R version 4.3.3 (2024-02-29)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Ubuntu 22.04.4 LTS
Matrix products: default
BLAS: /usr/lib/x86_64-linux-gnu/openblas-pthread/libblas.so.3
LAPACK: /usr/lib/x86_64-linux-gnu/openblas-pthread/libopenblasp-r0.3.20.so; LAPACK version 3.10.0
locale:
[1] LC_CTYPE=en_AU.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_AU.UTF-8 LC_COLLATE=en_AU.UTF-8
[5] LC_MONETARY=en_AU.UTF-8 LC_MESSAGES=en_AU.UTF-8
[7] LC_PAPER=en_AU.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_AU.UTF-8 LC_IDENTIFICATION=C
time zone: Etc/UTC
tzcode source: system (glibc)
attached base packages:
[1] stats4 stats graphics grDevices datasets utils methods
[8] base
other attached packages:
[1] here_1.0.1 tidyHeatmap_1.8.1
[3] paletteer_1.6.0 patchwork_1.2.0
[5] speckle_1.2.0 glue_1.7.0
[7] org.Hs.eg.db_3.18.0 AnnotationDbi_1.64.1
[9] clustree_0.5.1 ggraph_2.2.0
[11] dittoSeq_1.14.2 glmGamPoi_1.14.3
[13] SeuratObject_4.1.4 Seurat_4.4.0
[15] lubridate_1.9.3 forcats_1.0.0
[17] stringr_1.5.1 dplyr_1.1.4
[19] purrr_1.0.2 readr_2.1.5
[21] tidyr_1.3.1 tibble_3.2.1
[23] ggplot2_3.5.0 tidyverse_2.0.0
[25] edgeR_4.0.15 limma_3.58.1
[27] SingleCellExperiment_1.24.0 SummarizedExperiment_1.32.0
[29] Biobase_2.62.0 GenomicRanges_1.54.1
[31] GenomeInfoDb_1.38.6 IRanges_2.36.0
[33] S4Vectors_0.40.2 BiocGenerics_0.48.1
[35] MatrixGenerics_1.14.0 matrixStats_1.2.0
[37] workflowr_1.7.1
loaded via a namespace (and not attached):
[1] fs_1.6.3 spatstat.sparse_3.0-3 bitops_1.0-7
[4] httr_1.4.7 RColorBrewer_1.1-3 doParallel_1.0.17
[7] dynamicTreeCut_1.63-1 backports_1.4.1 tools_4.3.3
[10] sctransform_0.4.1 utf8_1.2.4 R6_2.5.1
[13] lazyeval_0.2.2 uwot_0.1.16 GetoptLong_1.0.5
[16] withr_3.0.0 sp_2.1-3 gridExtra_2.3
[19] preprocessCore_1.64.0 progressr_0.14.0 WGCNA_1.72-5
[22] cli_3.6.2 spatstat.explore_3.2-6 labeling_0.4.3
[25] sass_0.4.8 spatstat.data_3.0-4 ggridges_0.5.6
[28] pbapply_1.7-2 foreign_0.8-86 sessioninfo_1.2.2
[31] parallelly_1.37.0 impute_1.76.0 rstudioapi_0.15.0
[34] RSQLite_2.3.5 generics_0.1.3 shape_1.4.6
[37] ica_1.0-3 spatstat.random_3.2-2 dendextend_1.17.1
[40] GO.db_3.18.0 Matrix_1.6-5 fansi_1.0.6
[43] abind_1.4-5 lifecycle_1.0.4 whisker_0.4.1
[46] yaml_2.3.8 SparseArray_1.2.4 Rtsne_0.17
[49] grid_4.3.3 blob_1.2.4 promises_1.2.1
[52] crayon_1.5.2 miniUI_0.1.1.1 lattice_0.22-5
[55] cowplot_1.1.3 KEGGREST_1.42.0 pillar_1.9.0
[58] knitr_1.45 ComplexHeatmap_2.18.0 rjson_0.2.21
[61] future.apply_1.11.1 codetools_0.2-19 leiden_0.4.3.1
[64] getPass_0.2-4 data.table_1.15.0 vctrs_0.6.5
[67] png_0.1-8 gtable_0.3.4 rematch2_2.1.2
[70] cachem_1.0.8 xfun_0.42 S4Arrays_1.2.0
[73] mime_0.12 tidygraph_1.3.1 survival_3.7-0
[76] pheatmap_1.0.12 iterators_1.0.14 statmod_1.5.0
[79] ellipsis_0.3.2 fitdistrplus_1.1-11 ROCR_1.0-11
[82] nlme_3.1-164 bit64_4.0.5 RcppAnnoy_0.0.22
[85] rprojroot_2.0.4 bslib_0.6.1 irlba_2.3.5.1
[88] rpart_4.1.23 KernSmooth_2.23-24 Hmisc_5.1-1
[91] colorspace_2.1-0 DBI_1.2.1 nnet_7.3-19
[94] tidyselect_1.2.0 processx_3.8.3 bit_4.0.5
[97] compiler_4.3.3 git2r_0.33.0 htmlTable_2.4.2
[100] DelayedArray_0.28.0 plotly_4.10.4 checkmate_2.3.1
[103] scales_1.3.0 lmtest_0.9-40 callr_3.7.3
[106] digest_0.6.34 goftest_1.2-3 spatstat.utils_3.0-4
[109] rmarkdown_2.25 XVector_0.42.0 base64enc_0.1-3
[112] htmltools_0.5.7 pkgconfig_2.0.3 highr_0.10
[115] fastmap_1.1.1 rlang_1.1.3 GlobalOptions_0.1.2
[118] htmlwidgets_1.6.4 shiny_1.8.0 farver_2.1.1
[121] jquerylib_0.1.4 zoo_1.8-12 jsonlite_1.8.8
[124] RCurl_1.98-1.14 magrittr_2.0.3 Formula_1.2-5
[127] GenomeInfoDbData_1.2.11 munsell_0.5.0 Rcpp_1.0.12
[130] viridis_0.6.5 reticulate_1.35.0 stringi_1.8.3
[133] zlibbioc_1.48.0 MASS_7.3-60.0.1 plyr_1.8.9
[136] parallel_4.3.3 listenv_0.9.1 ggrepel_0.9.5
[139] deldir_2.0-2 Biostrings_2.70.2 graphlayouts_1.1.0
[142] splines_4.3.3 tensor_1.5 hms_1.1.3
[145] circlize_0.4.15 locfit_1.5-9.8 ps_1.7.6
[148] fastcluster_1.2.6 igraph_2.0.1.1 spatstat.geom_3.2-8
[151] reshape2_1.4.4 evaluate_0.23 renv_1.0.3
[154] BiocManager_1.30.22 tzdb_0.4.0 foreach_1.5.2
[157] tweenr_2.0.3 httpuv_1.6.14 RANN_2.6.1
[160] polyclip_1.10-6 future_1.33.1 clue_0.3-65
[163] scattermore_1.2 ggforce_0.4.2 xtable_1.8-4
[166] later_1.3.2 viridisLite_0.4.2 memoise_2.0.1
[169] cluster_2.1.6 timechange_0.3.0 globals_0.16.2